Purification and Characterization of a Unique Pectin Lyase from Aspergillus giganteus Able to Release Unsaturated Monogalacturonate during Pectin Degradation
- PMID: 25610636
- PMCID: PMC4294307
- DOI: 10.1155/2014/353915
Purification and Characterization of a Unique Pectin Lyase from Aspergillus giganteus Able to Release Unsaturated Monogalacturonate during Pectin Degradation
Abstract
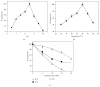
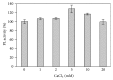
A pectin lyase, named PLIII, was purified to homogeneity from the culture filtrate of Aspergillus giganteus grown in submerged culture containing orange peel waste as carbon source. PLIII was able to digest apple pectin and citrus pectins with different degrees of methyl esterification. Interestingly, the PLIII activity was stimulated in the presence of some divalent cations including Pb(2+) and was not significantly affected by Hg(2+). Like other pectin lyases, PLIII is stimulated by but is not dependent on Ca(2+). The main soluble product released during the degradation of pectic substances promoted by the PLIII is compatible with an unsaturated monogalacturonate. PLIII is a unique enzyme able to release unsaturated monogalacturonate as the only soluble product during the degradation of pectic substances; therefore, PLIII was classified as an exo-pectin lyase. To our knowledge, this is the first characterization of an exo-pectin lyase. The PLIII described in this work is potentially useful for ethanol production from pectin-rich biomass, besides other common applications for alkaline pectinases like preparation of textile fibers, coffee and tea fermentation, vegetable oil extraction, and the treatment of pulp in papermaking.
Figures



References
-
- Gummadi S. N., Panda T. Purification and biochemical properties of microbial pectinases—a review. Process Biochemistry. 2003;38(7):987–996. doi: 10.1016/s0032-9592(02)00203-0. - DOI
-
- Jayani R. S., Saxena S., Gupta R. Microbial pectinolytic enzymes: a review. Process Biochemistry. 2005;40(9):2931–2944. doi: 10.1016/j.procbio.2005.03.026. - DOI
-
- Edwards M. C., Henriksen E. D., Yomano L. P., et al. Addition of genes for cellobiase and pectinolytic activity in Escherichia coli for fuel ethanol production from pectin-rich lignocellulosic biomass. Applied and Environmental Microbiology. 2011;77(15):5184–5191. doi: 10.1128/aem.05700-11. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

