Human polymorphonuclear neutrophils specifically recognize and kill cancerous cells
- PMID: 25610737
- PMCID: PMC4292216
- DOI: 10.4161/15384101.2014.950163
Human polymorphonuclear neutrophils specifically recognize and kill cancerous cells
Abstract
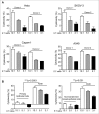
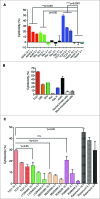
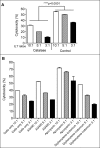
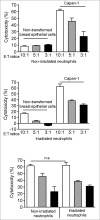
Polymorphonuclear neutrophils (PMNs), the main effectors of the innate immune system, have rarely been considered as an anticancer therapeutic tool. However, recent investigations using animal models and preliminary clinical studies have highlighted the potential antitumor efficacy of PMNs. In the current study, we find that PMNs from some healthy donors naturally have potent cancer-killing activity against 4 different human cancer cell lines. The killing activity appears to be cancer cell-specific since PMNs did not kill primary normal epithelial cells or an immortalized breast epithelial cell line. Transfecting the immortalized mammary cells with plasmids expressing activated forms of the rat sarcoma viral oncogene homolog (Ras) and teratocarcinoma oncogene 21 (TC21) oncogenes was sufficient to provoke aggressive attack by PMNs. However, transfection with activated Ras-related C3 botulinum toxin substrate (Rac1) was ineffective, suggesting specificity in PMN-targeting of neoplastic cells. Furthermore, PMNs from lung cancer patients were also found to exhibit relatively poor cancer-killing activity compared to the cytolytic activity of the average healthy donor. Taken together, our results suggest that PMN-based treatment regimens may represent a paradigm shift in cancer immunotherapy that may be easily introduced into the clinic to benefit a subset of patients with PMN-vulnerable tumors.
Keywords: BEN, benign ethnic neutropenia; DBL, proto-oncogene DBL; DPI, diphenyleneiodonium; E:T, effector:target; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte macrophage-colony stimulating factor; GVHD, graft-versus-host disease; H-Ras, Harvey rat sarcoma viral oncogene homolog; MEK, mitogen-activated protein kinase kinase; NADPH, nicotinamide adenine dinucleotide phosphate; NBT, nitroblue tetrazolium; NSCLC, non-small cell lung carcinoma; PI3 kinase, phosphoinositide 3-kinase; PMN, polymorphonuclear neutrophils; ROS, reactive oxygen species; Rac1, Ras-related C3 botulinum toxin substrate 1; RhoA, Ras homolog family member A; TC-21, teratocarcinoma oncogene TC21; TGFβ, transforming growth factor; cytotoxicity; mAb, monoclonal antibody; mTOR, mammalian target of rapamycin; neutrophils; oncogene; tumor cells.
Figures

References
-
- Nathan C. Neutrophils and immunity:challenges and opportunities. Nat Rev Immunol 2006; 6:173-82; PMID:; http://dx.doi.org/10.1038/nri1785 - DOI - PubMed
-
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 1989; 320:365-76; PMID:2536474 http://dx.doi.org/10.1056/NEJM198902093200606 - DOI - PubMed
-
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:; http://dx.doi.org/10.1038/nature07205 - DOI - PubMed
-
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860-7; PMID:; http://dx.doi.org/10.1038/nature01322 - DOI - PMC - PubMed
-
- Stoppacciaro A, Melani C, Parenza M, Mastracchio A, Bassi C, Baroni C, Parmiani G, Colombo MP. Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-T cell cooperation and T cell-produced interferon gamma. J Exp Med 1993; 178:151-61; PMID:; http://dx.doi.org/10.1084/jem.178.1.151 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
