Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis
- PMID: 25612136
- PMCID: PMC4303292
- DOI: 10.1371/journal.pmed.1001778
Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis
Abstract
Background: Observational studies of a putative association between hormonal contraception (HC) and HIV acquisition have produced conflicting results. We conducted an individual participant data (IPD) meta-analysis of studies from sub-Saharan Africa to compare the incidence of HIV infection in women using combined oral contraceptives (COCs) or the injectable progestins depot-medroxyprogesterone acetate (DMPA) or norethisterone enanthate (NET-EN) with women not using HC.
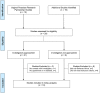
Methods and findings: Eligible studies measured HC exposure and incident HIV infection prospectively using standardized measures, enrolled women aged 15-49 y, recorded ≥15 incident HIV infections, and measured prespecified covariates. Our primary analysis estimated the adjusted hazard ratio (aHR) using two-stage random effects meta-analysis, controlling for region, marital status, age, number of sex partners, and condom use. We included 18 studies, including 37,124 women (43,613 woman-years) and 1,830 incident HIV infections. Relative to no HC use, the aHR for HIV acquisition was 1.50 (95% CI 1.24-1.83) for DMPA use, 1.24 (95% CI 0.84-1.82) for NET-EN use, and 1.03 (95% CI 0.88-1.20) for COC use. Between-study heterogeneity was mild (I(2) < 50%). DMPA use was associated with increased HIV acquisition compared with COC use (aHR 1.43, 95% CI 1.23-1.67) and NET-EN use (aHR 1.32, 95% CI 1.08-1.61). Effect estimates were attenuated for studies at lower risk of methodological bias (compared with no HC use, aHR for DMPA use 1.22, 95% CI 0.99-1.50; for NET-EN use 0.67, 95% CI 0.47-0.96; and for COC use 0.91, 95% CI 0.73-1.41) compared to those at higher risk of bias (p(interaction) = 0.003). Neither age nor herpes simplex virus type 2 infection status modified the HC-HIV relationship.
Conclusions: This IPD meta-analysis found no evidence that COC or NET-EN use increases women's risk of HIV but adds to the evidence that DMPA may increase HIV risk, underscoring the need for additional safe and effective contraceptive options for women at high HIV risk. A randomized controlled trial would provide more definitive evidence about the effects of hormonal contraception, particularly DMPA, on HIV risk.
Conflict of interest statement
NL is a member of the Editorial Board of PLOS Medicine. All other authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- 097410/WT_/Wellcome Trust/United Kingdom
- G0701039/MRC_/Medical Research Council/United Kingdom
- 1R21HD069192-01/HD/NICHD NIH HHS/United States
- G0100137/MRC_/Medical Research Council/United Kingdom
- R01 MH095507/MH/NIMH NIH HHS/United States
- G1002369/MRC_/Medical Research Council/United Kingdom
- MC_U122861322/MRC_/Medical Research Council/United Kingdom
- R21 HD069192/HD/NICHD NIH HHS/United States
- MC_UU_12023/1/MRC_/Medical Research Council/United Kingdom
- MR/K012126/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12023/23/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

