γδ T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-αβ+/CD19+ lymphocytes
- PMID: 25612623
- PMCID: PMC4440890
- DOI: 10.1182/blood-2014-09-599423
γδ T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-αβ+/CD19+ lymphocytes
Erratum in
- Blood. 2016 Mar 24;127(12):1620
-
Erratum: Airoldi I, Bertaina A, Prigione I, et al. γδ T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-αβ+/CD19+ lymphocytes. Blood. 2015;125(15):2349-2358.Blood. 2016 Mar 24;127(12):1620. doi: 10.1182/blood-2016-02-700625. Blood. 2016. PMID: 31265490 Free PMC article.
Abstract
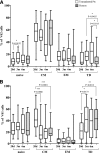
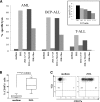
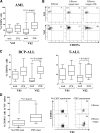
We prospectively assessed functional and phenotypic characteristics of γδ T lymphocytes up to 7 months after HLA-haploidentical hematopoietic stem cell transplantation (haplo-HSCT) depleted of αβ(+) T cells and CD19(+) B cells in 27 children with either malignant or nonmalignant disorders. We demonstrate that (1) γδ T cells are the predominant T-cell population in patients during the first weeks after transplantation, being mainly, albeit not only, derived from cells infused with the graft and expanding in vivo; (2) central-memory cells predominated very early posttransplantation for both Vδ1 and Vδ2 subsets; (3) Vδ1 cells are specifically expanded in patients experiencing cytomegalovirus reactivation and are more cytotoxic compared with those of children who did not experience reactivation; (4) these subsets display a cytotoxic phenotype and degranulate when challenged with primary acute myeloid and lymphoid leukemia blasts; and (5) Vδ2 cells are expanded in vitro after exposure to zoledronic acid (ZOL) and efficiently lyse primary lymphoid and myeloid blasts. This is the first detailed characterization of γδ T cells emerging in peripheral blood of children after CD19(+) B-cell and αβ(+) T-cell-depleted haplo-HSCT. Our results can be instrumental to the development of clinical trials using ZOL for improving γδ T-cell killing capacity against leukemia cells. This trial was registered at www.clinicaltrials.gov as #NCT01810120.
© 2015 by The American Society of Hematology.
Figures


Comment in
-
Haplo graft engineering: sculpting to a T.Blood. 2015 Apr 9;125(15):2315-6. doi: 10.1182/blood-2015-02-625269. Blood. 2015. PMID: 25858887
References
-
- Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339(17):1186–1193. - PubMed
-
- Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–3454. - PubMed
-
- Ciceri F, Labopin M, Aversa F, et al. Acute Leukemia Working Party (ALWP) of European Blood and Marrow Transplant (EBMT) Group. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008;112(9):3574–3581. - PubMed
-
- Klingebiel T, Cornish J, Labopin M, et al. Pediatric Diseases and Acute Leukemia Working Parties of the European Group for Blood and Marrow Transplantation (EBMT) Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood. 2010;115(17):3437–3446. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

