Being PRO-ACTive: What can a Clinical Trial Database Reveal About ALS?
- PMID: 25613183
- PMCID: PMC4404433
- DOI: 10.1007/s13311-015-0336-z
Being PRO-ACTive: What can a Clinical Trial Database Reveal About ALS?
Abstract
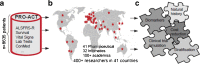
Advancing research and clinical care, and conducting successful and cost-effective clinical trials requires characterizing a given patient population. To gather a sufficiently large cohort of patients in rare diseases such as amyotrophic lateral sclerosis (ALS), we developed the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) platform. The PRO-ACT database currently consists of >8600 ALS patient records from 17 completed clinical trials, and more trials are being incorporated. The database was launched in an open-access mode in December 2012; since then, >400 researchers from >40 countries have requested the data. This review gives an overview on the research enabled by this resource, through several examples of research already carried out with the goal of improving patient care and understanding the disease. These examples include predicting ALS progression, the simulation of future ALS clinical trials, the verification of previously proposed predictive features, the discovery of novel predictors of ALS progression and survival, the newly identified stratification of patients based on their disease progression profiles, and the development of tools for better clinical trial recruitment and monitoring. Results from these approaches clearly demonstrate the value of large datasets for developing a better understanding of ALS natural history, prognostic factors, patient stratification, and more. The increasing use by the community suggests that further analyses of the PRO-ACT database will continue to reveal more information about this disease that has for so long defied our understanding.
Figures

References
-
- Miller R, Mitchell J, Lyon M, Moore D. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 2007;(1). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

