Pneumococcal vaccination during pregnancy for preventing infant infection
- PMID: 25613573
- PMCID: PMC9436174
- DOI: 10.1002/14651858.CD004903.pub4
Pneumococcal vaccination during pregnancy for preventing infant infection
Abstract
Background: Approximately 450,000 children worldwide die of pneumococcal infections each year. The development of bacterial resistance to antimicrobials adds to the difficulty of treatment of diseases and emphasizes the need for a preventive approach. Newborn vaccination schedules could substantially reduce the impact of pneumococcal disease in immunized children, but do not have an effect on the morbidity and mortality of infants less than three months of age. Pneumococcal vaccination during pregnancy may be a way of preventing pneumococcal disease during the first months of life before the pneumococcal vaccine administered to the infant starts to produce protection.
Objectives: To assess the effect of pneumococcal vaccination during pregnancy for preventing infant infection.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 July 2014) and reference lists of retrieved studies.
Selection criteria: Randomized controlled trials in pregnant women comparing pneumococcal vaccine with placebo or doing nothing, or with another vaccine to prevent infant infections.
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We contacted study authors for additional information.
Main results: Seven trials were included, but only six trials (919 participants) contributed data. There was no evidence that pneumococcal vaccination during pregnancy reduces the risk of neonatal infection (risk ratio (RR) 0.66; 95% confidence interval (CI) 0.30 to 1.46; two trials, 241 pregnancies, low quality evidence). Although the data suggest an effect in reducing pneumococcal colonization in infants by 16 months of age (average RR 0.33; 95% CI 0.11 to 0.98; one trial, 56 pregnancies), there was no evidence of this effect in infants at two to three months of age (average RR 1.13; 95% CI 0.46 to 2.78; two trials, 146 pregnancies, low quality evidence) or by six to seven months of age (average RR 0.67, 95% CI 0.22 to 2.08; two trials, 148 pregnancies, low quality evidence). None of the trials included in this review reported neonatal death as a result of pneumococcal infection.Neonatal antibody levels were reported as geometric mean and 95% CI. There were inconsistent results between studies. Two studies showed significantly higher immunoglobulin G (IgG) levels in cord blood in the pneumococcal vaccine group when compared with the control group for all serotypes. In contrast, another trial showed no difference in neonatal antibody levels between the pneumococcal vaccine group and the control group.Maternal antibody levels were also reported as geometric mean and 95% CI. One study showed significantly higher IgG levels in maternal serum in women immunized with pneumococcal vaccine when compared with control vaccine regardless of any serotypes. Another study showed significantly higher maternal antibody levels only for serotype 14, but no evidence of an effect for other serotypes.The percentage of women with seroprotection was measured in one trial at delivery and at 12 months post-delivery. At delivery, results favored the intervention group for serotype 6 (RR 1.49, 95% CI 1.31 to 1.69), serotype 14 (RR 1.40, 95% CI 1.25 to 1.56) and serotype 19 (RR 2.29, 95% CI 1.89 to 2.76). There were no group differences seen at 12 months post-delivery for serotypes 6 or 14 (RR 1.06, 95% CI 1.00 to 1.12 and RR 1.06, 95% CI 0.98 to 1.15, respectively), but results favored the intervention group for serotype 19 (RR 1.59, 95% CI 1.37 to 1.85).No significant difference for tenderness at the injection site between women who received pneumococcal vaccine and those who received control vaccine (average RR 3.20; 95% CI 0.32 to 31.54; two trials, 130 women).The overall quality of evidence is low for primary outcomes. Most outcomes had wide confidence intervals crossing the line of no effect, and most of the included trials had small numbers of participants and few events which led to downgrading evidence for imprecision of findings.
Authors' conclusions: There is insufficient evidence to assess whether pneumococcal vaccination during pregnancy could reduce infant infections.
Conflict of interest statement
None known.
Figures
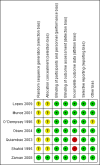




Update of
-
Pneumococcal vaccination during pregnancy for preventing infant infection.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD004903. doi: 10.1002/14651858.CD004903.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Jan 23;1:CD004903. doi: 10.1002/14651858.CD004903.pub4. PMID: 22786493 Updated.
References
References to studies included in this review
Lopes 2009 {published data only}
-
- Lopes CC, Berezin EN, Scheffer D, Huziwara R, Sliva MI, Brandao A, et al. Pneumococcal nasopharyngeal carriage in infants of mothers immunized with 23V non‐conjugate pneumococcal polysaccharide vaccine. Journal of Tropical Pediatrics 2012;58(5):348‐52. [PUBMED: 22238137] - PubMed
-
- Lopes CR, Berezin EN, Ching TH, Canuto Jde S, Costa VO, Klering EM. Ineffectiveness for infants of immunization of mothers with pneumococcal capsular polysaccharide vaccine during pregnancy. Brazilian Journal of Infectious Diseases 2009;13(2):104‐6. [PUBMED: 20140352] - PubMed
Munoz 2001 {published data only}
-
- Munoz FM, Englund JA, Cheesman CC, Maccato ML, Pinell PM, Nahm MH, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine 2001;20(5‐6):826‐37. [PUBMED: 11738746] - PubMed
O' Dempsey 1996 {published data only}
-
- O'Dempsey TJ, McArdle T, Ceesay SJ, Banya WA, Demba E, Secka O, et al. Immunization with a pneumococcal capsular polysaccharide vaccine during pregnancy. Vaccine 1996;14(10):963‐70. [PUBMED: 8873389] - PubMed
Obaro 2004 {published data only}
-
- Deubzer HE, Obaro SK, Newman VO, Adegbola RA, Greenwood BM, Henderson DC. Colostrum obtained from women vaccinated with pneumococcal vaccine during pregnancy inhibits epithelial adhesion of Streptococcus pneumoniae. Journal of Infectious Diseases 2004;190(10):1758‐61. [PUBMED: 15499530] - PubMed
-
- Obaro SK, Deubzer HE, Newman VO, Adegbola RA, Greenwood BM, Henderson DC. Serotype‐specific pneumococcal antibodies in breast milk of Gambian women immunized with a pneumococcal polysaccharide vaccine during pregnancy. Pediatric Infectious Disease Journal 2004;23(11):1023‐9. [PUBMED: 15545857] - PubMed
Quiambao 2003 {published data only}
-
- Holmlund E, Nohynek H, Quiambao B, Ollgren J, Kayhty H. Mother‐infant vaccination with pneumococcal polysaccharide vaccine: Persistence of maternal antibodies and responses of infants to vaccination. Vaccine 2011;29(28):4565‐75. [PUBMED: 21550374] - PubMed
-
- Quiambao BP, Nohynek H, Kayhty H, Ollgren J, Gozum L, Gepanayao CP, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the Philippines. Vaccine 2003;21(24):3451‐4. [PUBMED: 12850358] - PubMed
-
- Quiambao BP, Nohynek HM, Kayhty H, Ollgren JP, Gozum LS, Gepanayao CP, et al. Immunogenicity and reactogenicity of 23‐valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine 2007;25(22):4470‐7. [PUBMED: 17442467] - PubMed
Shahid 1995 {published data only}
-
- Shahid NS, Steinhoff MC, Hoque SS, Begum T, Thompson C, Siber GR. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet 1995;346(8985):1252‐7. [PUBMED: 7475716] - PubMed
Zaman 2008 {published data only}
-
- Henkle E, Steinhoff MC, Omer SB, Roy OE, Arifeen SE, Raqib R, et al. Incidence of influenza infection in early infancy in Southern Asia. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
-
- Omer SB, Zaman K, Roy E, Arifeen SE, Raqib R, Noory L, et al. Combined effects of antenatal receipt of influenza vaccine by mothers and pneumococcal conjugate vaccine receipt by infants: Results from a randomized, blinded, controlled trial. Journal of Infectious Diseases 2013;207(7):1144‐7. [PUBMED: 23300160] - PubMed
-
- Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Altaye M, et al. Influenza immunization in pregnancy and birth weights: observations from a randomized controlled trial. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
References to studies excluded from this review
Daly 2003 {published data only}
-
- Daly KA, Toth JA, Giebink GS. Pneumococcal conjugate vaccines as maternal and infant immunogens: challenges of maternal recruitment. Vaccine 2003;21(24):3473‐8. - PubMed
Glezen 2000 {published data only}
-
- Glezen W, Munoz F, Piedra P, Maccato M, Pinell P. Administration of pneumococcal polysaccharide vaccine (PCNP) and the purified fusion protein (PFP‐2) for respiratory syncytial virus to pregnant women. Infectious Diseases in Obstetrics & Gynecology 2000;8(3/4):200.
References to ongoing studies
Dunbar 2007 {published data only}
-
- Dunbar M, Moberley S, Nelson S, Leach AJ, Andrews R. Clear not simple: an approach to community consultation for a maternal pneumococcal vaccine trial among indigenous women in the Northern Territory of Australia. Vaccine 2007;25(13):2385‐8. - PubMed
Additional references
ACOG 2003
-
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion: immunization during pregnancy. Obstetrics & Gynecology 2003;101(1):207‐12. - PubMed
Arifeen 2009
Black 2000
-
- Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, et al. Efficacy safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatric Infectious Disease Journal 2000;19:187‐95. - PubMed
CDC 2000
-
- Centers for Disease Control and Prevention. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practice (ACIP). Morbidity and Mortality Weekly Report 2000;49(RR‐9):1‐29. - PubMed
CDC 2010
-
- Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children ‐ use of 13‐valent pneumococcal conjugate vaccine and 23‐valent pneumococcal polysaccharide vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 2010;59(RR‐11):1‐18. [PUBMED: 21150868] - PubMed
CDC 2011
-
- Centers for Disease Control and Prevention. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report 2011;60(RR‐2):26. [PUBMED: 21293327] - PubMed
Dagan 2000
-
- Dagan R, Fraser D. Conjugate pneumococcal vaccine and antibiotic‐resistant Streptococcus pneumoniae: herd immunity and reduction of otitis morbidity. Pediatric Infectious Diseases Journal 2000;19(5 Suppl):S79‐88. - PubMed
Foster 2008
-
- Foster D, Knox K, Walker AS, Griffiths DT, Moore H, Haworth E, et al. Invasive pneumococcal disease: epidemiology in children and adults prior to implementation of the conjugate vaccine in the Oxfordshire region, England. Journal of Medical Microbiology 2008;57(Pt 4):480‐7. - PubMed
GRADE 2008
-
- GRADEpro [Computer program]. Jan Brozek, Andrew Oxman, Holger Schünemann. Version 3.6 for Windows 2008.
Greenwood 2003
-
- Greenwood B. Maternal immunisation in developing countries. Vaccine 2003;21:3436‐41. - PubMed
Grijalva 2011
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Isaacman 2010
-
- Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7‐valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. International Journal of Infectious Diseases 2010;14(3):e197‐e209. [DOI: 10.1016/j.ijid.2009.05.010] - DOI - PubMed
Khan 2013
Klouwenberg 2008
Kono 2011
Lehmann 2002
-
- Lehmann D, Pomat WS, Combs B, Dyke T, Alpers MP. Maternal immunization with pneumococcal polysaccharide vaccine in the highlands of Papua New Guinea. Vaccine 2002;20(13‐14):1837–45. [PUBMED: 11906773] - PubMed
Pilishvili 2010
Pomat 2013
Rennels 1998
-
- Rennels MB, Edwards KM, Keyserling HL, Reisinger KS, Hogerman DA, Madore DV, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 1998;101:604‐11. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2009
-
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] - PubMed
Scott 2011
-
- Scott JA, Ojal J, Ashton L, Muhoro A, Burbidge P, Goldblatt D. Pneumococcal conjugate vaccine given shortly after birth stimulates effective antibody concentrations and primes immunological memory for sustained infant protection. Clinical Infectious Diseases 2011;53(7):663‐70. [DOI: 10.1093/cid/cir444] - DOI - PMC - PubMed
Whitney 2003
-
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein‐polysaccharide conjugate vaccine. New England Journal of Medicine 2003;348:1737‐46. - PubMed
WHO 2012
-
- World Health Organization. Pneumococcal vaccines WHO position paper ‐ 2012. Weekly Epidemiological Record 2012;87(14):129‐144. [PUBMED: 24340399]
References to other published versions of this review
Chaithongwongwatthana 2004
Chaithongwongwatthana 2006
Chaithongwongwatthana 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

