S100 proteins in cancer
- PMID: 25614008
- PMCID: PMC4369764
- DOI: 10.1038/nrc3893
S100 proteins in cancer
Abstract
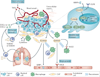
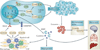
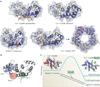
In humans, the S100 protein family is composed of 21 members that exhibit a high degree of structural similarity, but are not functionally interchangeable. This family of proteins modulates cellular responses by functioning both as intracellular Ca(2+) sensors and as extracellular factors. Dysregulated expression of multiple members of the S100 family is a common feature of human cancers, with each type of cancer showing a unique S100 protein profile or signature. Emerging in vivo evidence indicates that the biology of most S100 proteins is complex and multifactorial, and that these proteins actively contribute to tumorigenic processes such as cell proliferation, metastasis, angiogenesis and immune evasion. Drug discovery efforts have identified leads for inhibiting several S100 family members, and two of the identified inhibitors have progressed to clinical trials in patients with cancer. This Review highlights new findings regarding the role of S100 family members in cancer diagnosis and treatment, the contribution of S100 signalling to tumour biology, and the discovery and development of S100 inhibitors for treating cancer.
Figures

References
-
- Moore BW. A soluble protein characteristic of the nervous system. Biochem. Biophys. Res. Commun. 1965;19:739–744. - PubMed
-
- Leclerc E, Heizmann CW. The importance of Ca2+/ Zn2+ signaling S100 proteins and RAGE in translational medicine. Front. Biosci. (Schol. Ed.) 2011;3:1232–1262. - PubMed
-
- Yap KL, Ames JB, Swindells MB, Ikura M. Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins. 1999;37:499–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

