The role for adipose tissue in weight regain after weight loss
- PMID: 25614203
- PMCID: PMC4371661
- DOI: 10.1111/obr.12255
The role for adipose tissue in weight regain after weight loss
Abstract
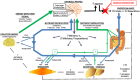
Weight regain after weight loss is a substantial challenge in obesity therapeutics. Dieting leads to significant adaptations in the homeostatic system that controls body weight, which promotes overeating and the relapse to obesity. In this review, we focus specifically on the adaptations in white adipose tissues that contribute to the biological drive to regain weight after weight loss. Weight loss leads to a reduction in size of adipocytes and this decline in size alters their metabolic and inflammatory characteristics in a manner that facilitates the clearance and storage of ingested energy. We present the hypothesis whereby the long-term signals reflecting stored energy and short-term signals reflecting nutrient availability are derived from the cellularity characteristics of adipose tissues. These signals are received and integrated in the hypothalamus and hindbrain and an energy gap between appetite and metabolic requirements emerges and promotes a positive energy imbalance and weight regain. In this paradigm, the cellularity and metabolic characteristics of adipose tissues after energy-restricted weight loss could explain the persistence of a biological drive to regain weight during both weight maintenance and the dynamic period of weight regain.
Keywords: Adipogenesis; dieting; obesity; weight regulation.
© 2015 The Authors. Obesity reviews © 2015 International Association for the Study of Obesity.
Figures

References
-
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. - PubMed
-
- Catenacci VA, Hill JO, Wyatt HR. The obesity epidemic. Clin Chest Med. 2009;30:415–444. - PubMed
-
- Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–584. - PubMed
-
- Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

