Fibrin, γ'-fibrinogen, and transclot pressure gradient control hemostatic clot growth during human blood flow over a collagen/tissue factor wound
- PMID: 25614284
- PMCID: PMC4344417
- DOI: 10.1161/ATVBAHA.114.305054
Fibrin, γ'-fibrinogen, and transclot pressure gradient control hemostatic clot growth during human blood flow over a collagen/tissue factor wound
Abstract
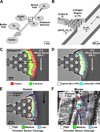
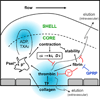
Objective: Biological and physical factors interact to modulate blood response in a wounded vessel, resulting in a hemostatic clot or an occlusive thrombus. Flow and pressure differential (ΔP) across the wound from the lumen to the extravascular compartment may impact hemostasis and the observed core/shell architecture. We examined physical and biological factors responsible for regulating thrombin-mediated clot growth.
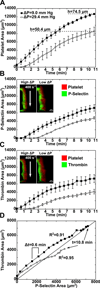
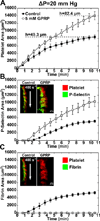
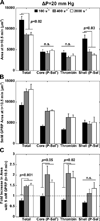
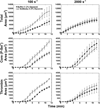
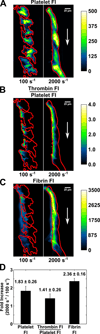
Approach and results: Using factor XIIa-inhibited human whole blood perfused in a microfluidic device over collagen/tissue factor at controlled wall shear rate and ΔP, we found thrombin to be highly localized in the P-selectin(+) core of hemostatic clots. Increasing ΔP from 9 to 29 mm Hg (wall shear rate=400 s(-1)) reduced P-selectin(+) core size and total clot size because of enhanced extravasation of thrombin. Blockade of fibrin polymerization with 5 mmol/L Gly-Pro-Arg-Pro dysregulated hemostasis by enhancing both P-selectin(+) core size and clot size at 400 s(-1) (20 mm Hg). For whole-blood flow (no Gly-Pro-Arg-Pro), the thickness of the P-selectin-negative shell was reduced under arterial conditions (2000 s(-1), 20 mm Hg). Consistent with the antithrombin-1 activity of fibrin implicated with Gly-Pro-Arg-Pro, anti-γ'-fibrinogen antibody enhanced core-localized thrombin, core size, and overall clot size, especially at venous (100 s(-1)) but not arterial wall shear rates (2000 s(-1)). Pathological shear (15 000 s(-1)) and Gly-Pro-Arg-Pro synergized to exacerbate clot growth.
Conclusions: Hemostatic clotting was dependent on core-localized thrombin that (1) triggered platelet P-selectin display and (2) was highly regulated by fibrin and the transclot ΔP. Also, γ'-fibrinogen had a role in venous but not arterial conditions.
Keywords: fibrin; hemodynamics; hemostasis; thrombin.
© 2015 American Heart Association, Inc.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

