Immune cell promotion of metastasis
- PMID: 25614318
- PMCID: PMC4470277
- DOI: 10.1038/nri3789
Immune cell promotion of metastasis
Abstract
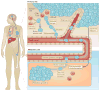
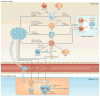
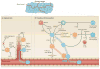
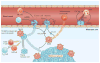
Metastatic disease is the major cause of death from cancer, and immunotherapy and chemotherapy have had limited success in reversing its progression. Data from mouse models suggest that the recruitment of immunosuppressive cells to tumours protects metastatic cancer cells from surveillance by killer cells, which nullifies the effects of immunotherapy and thus establishes metastasis. Furthermore, in most cases, tumour-infiltrating immune cells differentiate into cells that promote each step of the metastatic cascade and thus are novel targets for therapy. In this Review, we describe how tumour-infiltrating immune cells contribute to the metastatic cascade and we discuss potential therapeutic strategies to target these cells.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nature Rev Immunol. 2006;6:836–848. - PubMed
-
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. References 1 and 2 summarize the concept of tumour immune surveillance and immune escape. - PubMed
-
- Bidwell BN, et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nature Med. 2012;18:1224–1231. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

