Role of innate and adaptive immune mechanisms in cardiac injury and repair
- PMID: 25614321
- PMCID: PMC4669103
- DOI: 10.1038/nri3800
Role of innate and adaptive immune mechanisms in cardiac injury and repair
Abstract
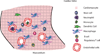
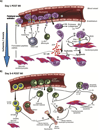
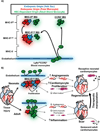
Despite the advances that have been made in developing new therapeutics, cardiovascular disease remains the leading cause of worldwide mortality. Therefore, understanding the mechanisms underlying cardiovascular tissue injury and repair is of prime importance. Following cardiac tissue injury, the immune system has an important and complex role in driving both the acute inflammatory response and the regenerative response. This Review summarizes the role of the immune system in cardiovascular disease - focusing on the idea that the immune system evolved to promote tissue homeostasis following injury and/or infection, and that the inherent cost of this evolutionary development is unwanted inflammatory damage.
Figures


References
-
- Bier E, Bodmer R. Drosophila, an emerging model for cardiac disease. Gene. 2004;342:1–11. - PubMed
-
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. - PubMed
-
- Laube F, Heister M, Scholz C, Borchardt T, Braun T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J. Cell Sci. 2006;119:4719–4729. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

