Experimental evaluation and computational modeling of tissue damage from low-flow push-pull perfusion sampling in vivo
- PMID: 25614385
- PMCID: PMC4331210
- DOI: 10.1016/j.jneumeth.2015.01.019
Experimental evaluation and computational modeling of tissue damage from low-flow push-pull perfusion sampling in vivo
Abstract
Background: Neurochemical monitoring via sampling probes is valuable for deciphering neurotransmission in vivo. Microdialysis is commonly used; however, the spatial resolution is poor.
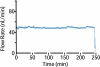
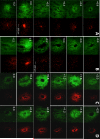
New method: Recently push-pull perfusion at low flow rates (50nL/min) has been proposed as a method for in vivo sampling from the central nervous system. Tissue damage from such probes has not been investigated in detail. In this work, we evaluated acute tissue response to low-flow push-pull perfusion by infusing the nuclear stains Sytox Orange and Hoechst 33342 through probes implanted in the striatum for 200min, to label damaged and total cells, respectively, in situ.
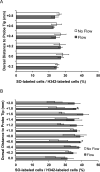
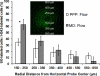
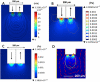
Results: Using the damaged/total labeled cell ratio as a measure of tissue damage, we found that 33±8% were damaged within the dye region around a microdialysis probe. We found that low-flow push-pull perfusion probes damaged 24±4% of cells in the sampling area. Flow had no effect on the number of damaged cells for low-flow push-pull perfusion. Modeling revealed that shear stress and pressure gradients generated by the flow were lower than thresholds expected to cause damage. Comparison with existing methods.Push-pull perfusion caused less tissue damage but yielded 1500-fold better spatial resolution.
Conclusions: Push-pull perfusion at low flow rates is a viable method for sampling from the brain with potential for high temporal and spatial resolution. Tissue damage is mostly caused by probe insertion. Smaller probes may yield even lower damage.
Keywords: Brain tissue damage; Cell viability; Computational modeling; In vivo sampling; Microdialysis; Push–pull perfusion.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

References
-
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Regional cerebral glucose phosphorylation and blood-flow after insertion of a microdialysis fiber through the dorsal hippocampus in the rat. Journal of Neurochemistry. 1987;49:729–34. - PubMed
-
- Cellar NA, Kennedy RT. A capillary-PDMS hybrid chip for separations-based sensing of neurotransmitters in vivo. Lab on a Chip. 2006;6:1205–12. - PubMed
-
- Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. Journal of Neuroscience Methods. 1999;90:129–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

