A systems-level approach to parental genomic imprinting: the imprinted gene network includes extracellular matrix genes and regulates cell cycle exit and differentiation
- PMID: 25614607
- PMCID: PMC4352888
- DOI: 10.1101/gr.175919.114
A systems-level approach to parental genomic imprinting: the imprinted gene network includes extracellular matrix genes and regulates cell cycle exit and differentiation
Abstract
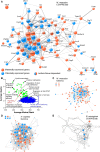
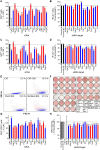
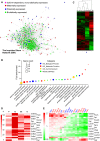
Genomic imprinting is an epigenetic mechanism that restrains the expression of ∼ 100 eutherian genes in a parent-of-origin-specific manner. The reason for this selective targeting of genes with seemingly disparate molecular functions is unclear. In the present work, we show that imprinted genes are coexpressed in a network that is regulated at the transition from proliferation to quiescence and differentiation during fibroblast cell cycle withdrawal, adipogenesis in vitro, and muscle regeneration in vivo. Imprinted gene regulation is not linked to alteration of DNA methylation or to perturbation of monoallelic, parent-of-origin-dependent expression. Overexpression and knockdown of imprinted gene expression alters the sensitivity of preadipocytes to contact inhibition and adipogenic differentiation. In silico and in cellulo experiments showed that the imprinted gene network includes biallelically expressed, nonimprinted genes. These control the extracellular matrix composition, cell adhesion, cell junction, and extracellular matrix-activated and growth factor-activated signaling. These observations show that imprinted genes share a common biological process that may account for their seemingly diverse roles in embryonic development, obesity, diabetes, muscle physiology, and neoplasm.
© 2015 Al Adhami et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
-
- Barlow DP. 2011. Genomic imprinting: a mammalian epigenetic discovery model. Annu Rev Genet 45: 379–403. - PubMed
-
- Bilanges B, Varrault A, Mazumdar A, Pantaloni C, Hoffmann A, Bockaert J, Spengler D, Journot L. 2001. Alternative splicing of the imprinted candidate tumor suppressor gene ZAC regulates its antiproliferative and DNA binding activities. Oncogene 20: 1246–1253. - PubMed
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
