Fluvastatin upregulates the α 1C subunit of CaV1.2 channel expression in vascular smooth muscle cells via RhoA and ERK/p38 MAPK pathways
- PMID: 25614710
- PMCID: PMC4295146
- DOI: 10.1155/2014/237067
Fluvastatin upregulates the α 1C subunit of CaV1.2 channel expression in vascular smooth muscle cells via RhoA and ERK/p38 MAPK pathways
Erratum in
-
Corrigendum to "Fluvastatin Upregulates the α 1C Subunit of CaV1.2 Channel Expression in Vascular Smooth Muscle Cells via RhoA and ERK/p38 MAPK Pathways".Dis Markers. 2024 Jun 22;2024:9875935. doi: 10.1155/2024/9875935. eCollection 2024. Dis Markers. 2024. PMID: 38948137 Free PMC article.
Abstract
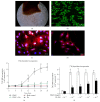
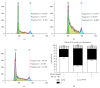
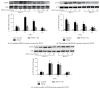
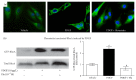
Abnormal phenotypic switch of vascular smooth muscle cell (VSMC) is a hallmark of vascular disorders such as atherosclerosis and restenosis. And this process has been related to remodeling of L-type calcium channel (LTCC). We attempted to investigate whether fluvastatin has any effect on VSMC proliferation and LTCCα 1C subunit (LTCCα 1C) expression as well as the potential mechanisms involved. The VSMCs proliferation was assayed by osteopontin immunofluorescent staining and [(3)H]-thymidine incorporation. The cell cycle was detected by flow cytometric analysis. The activity of RhoA was determined with pull-down assay. MAPK activity and LTCCα 1C expression were assessed by western blotting. We demonstrated fluvastatin prevented the VSMCs dedifferentiating into a proliferative phenotype and induced cell cycle arrest in the G0/G1 phase in response to PDGF-BB stimulation. Fluvastatin dose-dependently reversed the downregulation of LTCCα 1C expression induced by PDGF-BB. Inhibition of ROCK, ERK, or p38 MAPK activation largely enhanced the upregulation effect of fluvastatin (P < 0.01). However, blockade of JNK pathway had no effect on LTCCα 1C expression. We concluded LTCCα 1C was a VSMC contractile phenotype marker gene. Fluvastatin upregulated LTCCα 1C expression, at least in part, by inhibiting ROCK, ERK1/2, and p38 MAPK activation. Fluvastatin may be a potential candidate for preventing or treating vascular diseases.
Figures


Similar articles
-
TGF-beta1-induced thrombospondin-1 expression through the p38 MAPK pathway is abolished by fluvastatin in human coronary artery smooth muscle cells.Vascul Pharmacol. 2006 Jun;44(6):469-75. doi: 10.1016/j.vph.2006.03.002. Epub 2006 Apr 19. Vascul Pharmacol. 2006. PMID: 16624629
-
Fluvastatin enhances apoptosis in cytokine-stimulated vascular smooth muscle cells.J Cardiovasc Pharmacol. 2002 Feb;39(2):310-7. doi: 10.1097/00005344-200202000-00018. J Cardiovasc Pharmacol. 2002. PMID: 11791017
-
HMG-CoA reductase inhibitors reduce interleukin-6 synthesis in human vascular smooth muscle cells.Cardiovasc Drugs Ther. 2002 Mar;16(2):121-6. doi: 10.1023/a:1015701415588. Cardiovasc Drugs Ther. 2002. PMID: 12090904
-
RhoA/ROCK signaling regulates smooth muscle phenotypic modulation and vascular remodeling via the JNK pathway and vimentin cytoskeleton.Pharmacol Res. 2018 Jul;133:201-212. doi: 10.1016/j.phrs.2018.05.011. Epub 2018 May 20. Pharmacol Res. 2018. PMID: 29791873
-
Fluvastatin prevents vascular hyperplasia by inhibiting phenotype modulation and proliferation through extracellular signal-regulated kinase 1 and 2 and p38 mitogen-activated protein kinase inactivation in organ-cultured artery.Arterioscler Thromb Vasc Biol. 2005 Feb;25(2):327-33. doi: 10.1161/01.ATV.0000152611.50953.e2. Epub 2004 Dec 9. Arterioscler Thromb Vasc Biol. 2005. PMID: 15591221
Cited by
-
Elevated serum fatty acid-binding protein 4 level predicts all-cause and cardiovascular mortality in peritoneal dialysis patients: a five-year study.Ren Fail. 2023;45(2):2262624. doi: 10.1080/0886022X.2023.2262624. Epub 2023 Oct 2. Ren Fail. 2023. PMID: 37782286 Free PMC article.
-
Crocin prevents platelet‑derived growth factor BB‑induced vascular smooth muscle cells proliferation and phenotypic switch.Mol Med Rep. 2018 Jun;17(6):7595-7602. doi: 10.3892/mmr.2018.8854. Epub 2018 Apr 5. Mol Med Rep. 2018. PMID: 29620234 Free PMC article.
-
Effects of paclitaxel intervention on pulmonary vascular remodeling in rats with pulmonary hypertension.Exp Ther Med. 2019 Feb;17(2):1163-1170. doi: 10.3892/etm.2018.7045. Epub 2018 Dec 5. Exp Ther Med. 2019. PMID: 30679989 Free PMC article.
-
Corrigendum to "Fluvastatin Upregulates the α 1C Subunit of CaV1.2 Channel Expression in Vascular Smooth Muscle Cells via RhoA and ERK/p38 MAPK Pathways".Dis Markers. 2024 Jun 22;2024:9875935. doi: 10.1155/2024/9875935. eCollection 2024. Dis Markers. 2024. PMID: 38948137 Free PMC article.
-
Serum Amyloid A Induces a Vascular Smooth Muscle Cell Phenotype Switch through the p38 MAPK Signaling Pathway.Biomed Res Int. 2017;2017:4941379. doi: 10.1155/2017/4941379. Epub 2017 May 31. Biomed Res Int. 2017. PMID: 28642873 Free PMC article.
References
-
- Kudryavtseva O., Herum K. M., Dam V. S., et al. Downregulation of L-type Ca2+ channel in rat mesenteric arteries leads to loss of smooth muscle contractile phenotype and inward hypertrophic remodeling. The American Journal of Physiology—Heart and Circulatory Physiology. 2014;306(9):H1287–H1301. doi: 10.1152/ajpheart.00503.2013. - DOI - PubMed
-
- Okuda S., Yano M. Excitation-contraction coupling and intracellular calcium cycling in failing hearts. Clinical Calcium. 2013;23(4):471–480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

