Access to Formally Ni(I) States in a Heterobimetallic NiZn System
- PMID: 25614786
- PMCID: PMC4300139
- DOI: 10.1039/C2SC21231E
Access to Formally Ni(I) States in a Heterobimetallic NiZn System
Abstract
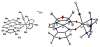
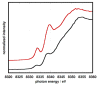
Heterobimetallic NiZn complexes featuring metal centers in distinct coordination environments have been synthesized using diimine-dioxime ligands as binucleating scaffolds. A tetramethylfuran-containing ligand derivative enables a stable one-electron-reduced S = 1/2 species to be accessed using Cp2Co as a chemical reductant. The resulting pseudo-square planar complex exhibits spectroscopic and crystallographic characteristics of a ligand-centered radical bound to a Ni(II) center. Upon coordination of a π-acidic ligand such as PPh3, however, a five-coordinate Ni(I) metalloradical is formed. The electronic structures of these reduced species provide insight into the subtle effects of ligand structure on the potential and reversibility of the NiII/I couple for complexes of redox-active tetraazamacrocycles.
Figures
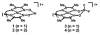
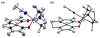









References
-
- Jeoung JH, Dobbek H. Science. 2007;318:1461–1464. - PubMed
-
- Li Z, Ohki Y, Tatsumi K. J Am Chem Soc. 2005;127:8950–8951. - PubMed
- Barton BE, Whaley CM, Rauchfuss TB, Gray DL. J Am Chem Soc. 2009;131:6942–6943. - PMC - PubMed
- Park YJ, Ziller JW, Borovik AS. J Am Chem Soc. 2011;133:9258–9261. - PMC - PubMed
- Fachinetti G, Floriani C, Zanazzi PF. J Am Chem Soc. 1978;100:7405–7407.
- Gambarotta S, Arena F, Floriani C, Zanazzi PF. J Am Chem Soc. 1982;104:5082–5092.
- Krogman JP, Foxman BM, Thomas CM. J Am Chem Soc. 2011;133:14582–14585. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

