Ipilimumab-induced autoimmune hypophysitis: a differential for sellar mass lesions
- PMID: 25614822
- PMCID: PMC4276072
- DOI: 10.1530/EDM-14-0098
Ipilimumab-induced autoimmune hypophysitis: a differential for sellar mass lesions
Abstract
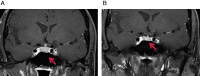
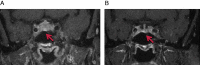
Autoimmune hypophysitis (AH) has been previously described in a typical demographic population, primarily women in the reproductive age group and perinatal period. The era of immune modulation using anti-cytotoxic T-lymphocyte-associated antigen 4 biological therapy (ipilimumab) against advanced cancers like metastatic melanomas has now resulted in a new form of hypophysitis being increasingly recognised under a spectrum of immune-related adverse events. Drug-related AH often presents with subtle symptoms and a pituitary mass, with the potential for fatality necessitating wide awareness and a high index of clinical suspicion given that it is usually treatable. We describe below two cases of AH within the last three months at our centre, which were treated with different regimens and produced good endocrine outcomes.
Learning points: AH is a new and defined clinical entity occurring as a side effect of ipilimumab, which enhances immune-mediated destruction of metastatic melanoma.It can present insidiously and have life-threatening complications related to hypocortisolism, hence a high index of clinical suspicion must be exerted by treating physicians, and seems to result in resolution of pituitary masses and variable improvements of pituitary function.Clinical improvement, radiological resolution of pituitary masses and variable normalisation of pituitary function are possible with early treatment with high-dose oral or i.v. steroids and hormone replacement therapy, although duration and dosing protocols are unclear at this stage.Ipilimumab should continue to be prescribed as treatment for metastatic melanoma; however, close clinical observation of patient's progress must be maintained while they are on this drug.Predictive factors for onset of AH remain unclear and it is imperative that AH is distinguished from pituitary metastases.Further studies are required to determine the safety of continuing therapy with ipilimumab in patients who have developed AH while on treatment.
Figures
References
-
- Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis Set al. 2007Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. Journal of Immunotherapy 30825–830 10.1097/CJI.0b013e318156e47e - DOI - PMC - PubMed
-
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CLet al. 2007Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clinical Cancer Research 136681–6688 10.1158/1078-0432.CCR-07-0187 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

