All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study
- PMID: 25614962
- PMCID: PMC4409820
- DOI: 10.1002/hep.27726
All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study
Abstract
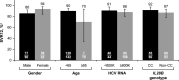
Treatment options for patients with hepatitis C virus (HCV) genotype 3 infection are limited, with the currently approved all-oral regimens requiring 24-week treatment and the addition of ribavirin (RBV). This phase III study (ALLY-3; ClinicalTrials.gov: NCT02032901) evaluated the 12-week regimen of daclatasvir (DCV; pangenotypic nonstructural protein [NS]5A inhibitor) plus sofosbuvir (SOF; pangenotypic NS5B inhibitor) in patients infected with genotype 3. Patients were either treatment naïve (n = 101) or treatment experienced (n = 51) and received DCV 60 mg plus SOF 400 mg once-daily for 12 weeks. Coprimary endpoints were the proportions of treatment-naïve and treatment-experienced patients achieving a sustained virological response (SVR) at post-treatment week 12 (SVR12). SVR12 rates were 90% (91 of 101) and 86% (44 of 51) in treatment-naïve and treatment-experienced patients, respectively; no virological breakthrough was observed, and ≥99% of patients had a virological response (VR) at the end of treatment. SVR12 rates were higher in patients without cirrhosis (96%; 105 of 109) than in those with cirrhosis (63%; 20 of 32). Five of seven patients who previously failed treatment with an SOF-containing regimen and 2 of 2 who previously failed treatment with an alisporivir-containing regimen achieved SVR12. Baseline characteristics, including gender, age, HCV-RNA levels, and interleukin-28B genotype, did not impact virological outcome. DCV plus SOF was well tolerated; there were no adverse events (AEs) leading to discontinuation and only 1 serious AE on-treatment, which was unrelated to study medications. The few treatment-emergent grade 3/4 laboratory abnormalities that were observed were transient.
Conclusion: A 12-week regimen of DCV plus SOF achieved SVR12 in 96% of patients with genotype 3 infection without cirrhosis and was well tolerated. Additional evaluation to optimize efficacy in genotype 3-infected patients with cirrhosis is underway.
© 2015 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures

Comment in
-
Daclatasvir plus sofosbuvir regimen sheds promising light on future hepatitis C virus genotype 3 therapies.Turk J Gastroenterol. 2016 Jan;27(1):89-90. doi: 10.5152/tjg.2015.150012. Turk J Gastroenterol. 2016. PMID: 26728866 No abstract available.
-
No Time for Bullies in Hepatitis C Virus Therapy: Genotype 3 Conquered?Gastroenterology. 2016 May;150(5):1241-1243. doi: 10.1053/j.gastro.2016.03.025. Epub 2016 Mar 24. Gastroenterology. 2016. PMID: 27018182 No abstract available.
References
-
- Pol S, Vallet-Pichard A, Corouge M. Treatment of hepatitis C virus genotype 3-infection. Liver Int. 2014;34(Suppl 1):18–23. - PubMed
-
- Negro F, Alberti A. The global health burden of hepatitis C virus infection. Liver Int. 2011;31(Suppl 2):1–3. - PubMed
-
- Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18:e516–e522. - PubMed
-
- Larsen C, Bousquet V, Delarocque-Astagneau E, Pioche C, Roudot-Thoraval F, et al. HCV Surveillance Steering Committee Hepatitis C virus genotype 3 and the risk of severe liver disease in a large population of drug users in France. J Med Virol. 2010;82:1647–1654. - PubMed
-
- Bochud PY, Cai T, Overbeck K, Bochud M, Dufour JF, Mullhaupt B, et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009;51:655–666. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
