Hydrogen sulfide donor protects porcine oocytes against aging and improves the developmental potential of aged porcine oocytes
- PMID: 25615598
- PMCID: PMC4304783
- DOI: 10.1371/journal.pone.0116964
Hydrogen sulfide donor protects porcine oocytes against aging and improves the developmental potential of aged porcine oocytes
Abstract
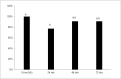
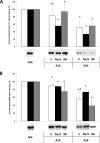
Porcine oocytes that have matured in in vitro conditions undergo the process of aging during prolonged cultivation, which is manifested by spontaneous parthenogenetic activation, lysis or fragmentation of aged oocytes. This study focused on the role of hydrogen sulfide (H2S) in the process of porcine oocyte aging. H2S is a gaseous signaling molecule and is produced endogenously by the enzymes cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (MPST). We demonstrated that H2S-producing enzymes are active in porcine oocytes and that a statistically significant decline in endogenous H2S production occurs during the first day of aging. Inhibition of these enzymes accelerates signs of aging in oocytes and significantly increases the ratio of fragmented oocytes. The presence of exogenous H2S from a donor (Na2S.9H2O) significantly suppressed the manifestations of aging, reversed the effects of inhibitors and resulted in the complete suppression of oocyte fragmentation. Cultivation of aging oocytes in the presence of H2S donor positively affected their subsequent embryonic development following parthenogenetic activation. Although no unambiguous effects of exogenous H2S on MPF and MAPK activities were detected and the intracellular mechanism underlying H2S activity remains unclear, our study clearly demonstrates the role of H2S in the regulation of porcine oocyte aging.
Conflict of interest statement
Figures




References
-
- Petrova I, Sedmikova M, Petr J, Vodkova Z, Pytloun P, et al. (2009) The role of c-Jun N-terminal kinase (JNK) and p38 Mitogen-activated protein kinase (p38 MAPK) in aged pig oocytes. J Reprod Dev, 55, 75–82. - PubMed
-
- Wang QL, Sun QY (2007) Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev 19(1):1–12. - PubMed
-
- Whitaker M (1996) Control of meiotic arrest. Reviews of Reproduction 1: 127–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

