The NF-κB regulator Bcl-3 modulates inflammation during contact hypersensitivity reactions in radioresistant cells
- PMID: 25616060
- PMCID: PMC4432939
- DOI: 10.1002/eji.201444994
The NF-κB regulator Bcl-3 modulates inflammation during contact hypersensitivity reactions in radioresistant cells
Abstract
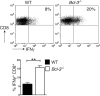
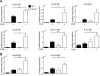
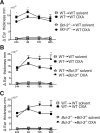
Bcl-3 is an atypical member of the IκB family. Bcl-3 functions as a cofactor of p50/NF-κB1 or p52/NF-κB2 homodimers in nuclei, where it modulates NF-κB-regulated transcription in a context-dependent way. Bcl-3 has tumorigenic potential, is critical in host defense of pathogens, and has been reported to ameliorate or exacerbate inflammation, depending on disease model. However, cell-specific functions of Bcl-3 remain largely unknown. Here, we explored the role of Bcl-3 in a contact hypersensitivity (CHS) mouse model, which depends on the interplay between keratinocytes and immune cells. Bcl-3-deficient mice exhibited an exacerbated and prolonged CHS response to oxazolone. Increased inflammation correlated with higher production of chemokines CXCL2, CXCL9, and CXCL10, and consequently increased recruitment of neutrophils and CD8(+) T cells. BM chimera experiments indicated that the ability of Bcl-3 to reduce the CHS response depended on Bcl-3 activity in radioresistant cells. Specific ablation of Bcl-3 in keratinocytes resulted in increased production of CXCL9 and CXCL10 and sustained recruitment of specifically CD8(+) T cells. These findings identify Bcl-3 as a critical player during the later stage of the CHS reaction to limit inflammation via actions in radioresistant cells, including keratinocytes.
Keywords: Bcl-3; Chemokines; Contact hypersensitivity; Keratinocytes; NF-κB.
Published 2015. This article is a U.S. Government work and is in the public domain in the USA.
Figures



References
-
- Christensen AD, Haase C. Immunological mechanisms of contact hypersensitivity in mice. APMIS. 2012;120:1–27. - PubMed
-
- Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol. 2013;133:303–315. - PubMed
-
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. - PubMed
-
- Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol. 2005;5:435–445. - PubMed
-
- Ohno H, Takimoto G, McKeithan TW. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990;60:991–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

