The ADP/ATP carrier and its relationship to oxidative phosphorylation in ancestral protist trypanosoma brucei
- PMID: 25616281
- PMCID: PMC4346568
- DOI: 10.1128/EC.00238-14
The ADP/ATP carrier and its relationship to oxidative phosphorylation in ancestral protist trypanosoma brucei
Abstract
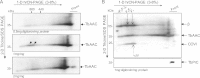
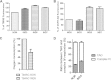
The highly conserved ADP/ATP carrier (AAC) is a key energetic link between the mitochondrial (mt) and cytosolic compartments of all aerobic eukaryotic cells, as it exchanges the ATP generated inside the organelle for the cytosolic ADP. Trypanosoma brucei, a parasitic protist of medical and veterinary importance, possesses a single functional AAC protein (TbAAC) that is related to the human and yeast ADP/ATP carriers. However, unlike previous studies performed with these model organisms, this study showed that TbAAC is most likely not a stable component of either the respiratory supercomplex III+IV or the ATP synthasome but rather functions as a physically separate entity in this highly diverged eukaryote. Therefore, TbAAC RNA interference (RNAi) ablation in the insect stage of T. brucei does not impair the activity or arrangement of the respiratory chain complexes. Nevertheless, RNAi silencing of TbAAC caused a severe growth defect that coincides with a significant reduction of mt ATP synthesis by both substrate and oxidative phosphorylation. Furthermore, TbAAC downregulation resulted in a decreased level of cytosolic ATP, a higher mt membrane potential, an elevated amount of reactive oxygen species, and a reduced consumption of oxygen in the mitochondria. Interestingly, while TbAAC has previously been demonstrated to serve as the sole ADP/ATP carrier for ADP influx into the mitochondria, our data suggest that a second carrier for ATP influx may be present and active in the T. brucei mitochondrion. Overall, this study provides more insight into the delicate balance of the functional relationship between TbAAC and the oxidative phosphorylation (OXPHOS) pathway in an early diverged eukaryote.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

