Marine fish oils are not equivalent with respect to B-cell membrane organization and activation
- PMID: 25616447
- PMCID: PMC4391512
- DOI: 10.1016/j.jnutbio.2014.11.005
Marine fish oils are not equivalent with respect to B-cell membrane organization and activation
Abstract
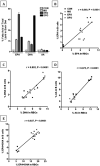
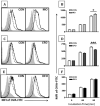
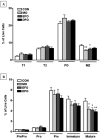
We previously reported that docosahexaenoic-acid (DHA)-enriched fish oil (DFO) feeding altered B-cell membrane organization and enhanced B-cell function. The purpose of this study was to evaluate whether menhaden oil (MO) and eicosapentaenoic-acid (EPA)-enriched fish oil (EFO) alters B-cell function/phenotype similarly. Mice were fed control (CON), MO, EFO or DFO diets for 5weeks. We evaluated the fatty acid composition of B-cell phospholipids, membrane microdomain organization, ex vivo B-cell functionality and in vivo B-cell subsets. Red blood cells and B cells were found to be strongly (r>0.85) and significantly (P<.001) correlated for major n-3 and n-6 long-chain polyunsaturated fatty acids (LCPUFAs). Compared to CON, MO and DFO resulted in decreased clustering of membrane microdomains, whereas EFO increased clustering. All fish oil treatments had 1.12-1.60 times higher CD40 expression following stimulation; however, we observed 0.86 times lower major histocompatibility complex class II expression and 0.7 times lower interleukin (IL)-6 production from EFO, but 3.25 times higher interferon-γ from MO and 1.5 times higher IL-6 from DFO. By 90min of incubation, MO had 1.11 times higher antigen uptake compared to CON, whereas EFO was 0.86 times lower. All fish oil treatments resulted in decreasingly mature splenic and bone marrow B-cell subsets. We conclude that diets high in n-3 LCPUFAs may elicit similar B-cell phenotypes but different organizational and functional outcomes. More specifically, these data suggest that the EPA and DHA content of a diet influences immunological outcomes, highlighting the importance of understanding how specific n-3 LCPUFAs modulate B-cell development and function.
Keywords: B cell; Fish oil; Immunomodulation; Inflammation; Membrane.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1–23. - PubMed
-
- Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

