Long-term treatment with responsive brain stimulation in adults with refractory partial seizures
- PMID: 25616485
- PMCID: PMC4339127
- DOI: 10.1212/WNL.0000000000001280
Long-term treatment with responsive brain stimulation in adults with refractory partial seizures
Abstract
Objective: The long-term efficacy and safety of responsive direct neurostimulation was assessed in adults with medically refractory partial onset seizures.
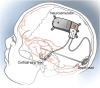
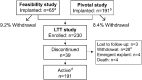
Methods: All participants were treated with a cranially implanted responsive neurostimulator that delivers stimulation to 1 or 2 seizure foci via chronically implanted electrodes when specific electrocorticographic patterns are detected (RNS System). Participants had completed a 2-year primarily open-label safety study (n = 65) or a 2-year randomized blinded controlled safety and efficacy study (n = 191); 230 participants transitioned into an ongoing 7-year study to assess safety and efficacy.
Results: The average participant was 34 (±11.4) years old with epilepsy for 19.6 (±11.4) years. The median preimplant frequency of disabling partial or generalized tonic-clonic seizures was 10.2 seizures a month. The median percent seizure reduction in the randomized blinded controlled trial was 44% at 1 year and 53% at 2 years (p < 0.0001, generalized estimating equation) and ranged from 48% to 66% over postimplant years 3 through 6 in the long-term study. Improvements in quality of life were maintained (p < 0.05). The most common serious device-related adverse events over the mean 5.4 years of follow-up were implant site infection (9.0%) involving soft tissue and neurostimulator explantation (4.7%).
Conclusions: The RNS System is the first direct brain responsive neurostimulator. Acute and sustained efficacy and safety were demonstrated in adults with medically refractory partial onset seizures arising from 1 or 2 foci over a mean follow-up of 5.4 years. This experience supports the RNS System as a treatment option for refractory partial seizures.
Classification of evidence: This study provides Class IV evidence that for adults with medically refractory partial onset seizures, responsive direct cortical stimulation reduces seizures and improves quality of life over a mean follow-up of 5.4 years.
© 2015 American Academy of Neurology.
Figures
Comment in
-
Stimulating the brain for epilepsy.Neurology. 2015 Feb 24;84(8):768-9. doi: 10.1212/WNL.0000000000001297. Epub 2015 Jan 23. Neurology. 2015. PMID: 25616484 No abstract available.
References
-
- Morrell MJ; RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77:1295–1304. - PubMed
-
- The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 1995;45:224–230. - PubMed
-
- Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology 1998;51:48–55. - PubMed
-
- Morris GL, III, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy: The Vagus Nerve Stimulation Study Group E01–E05. Neurology 1999;53:1731–1735. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
