Generation of a poor prognostic chronic lymphocytic leukemia-like disease model: PKCα subversion induces up-regulation of PKCβII expression in B lymphocytes
- PMID: 25616575
- PMCID: PMC4380723
- DOI: 10.3324/haematol.2014.112276
Generation of a poor prognostic chronic lymphocytic leukemia-like disease model: PKCα subversion induces up-regulation of PKCβII expression in B lymphocytes
Abstract
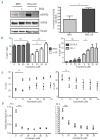
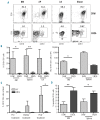
Overwhelming evidence identifies the microenvironment as a critical factor in the development and progression of chronic lymphocytic leukemia, underlining the importance of developing suitable translational models to study the pathogenesis of the disease. We previously established that stable expression of kinase dead protein kinase C alpha in hematopoietic progenitor cells resulted in the development of a chronic lymphocytic leukemia-like disease in mice. Here we demonstrate that this chronic lymphocytic leukemia model resembles the more aggressive subset of chronic lymphocytic leukemia, expressing predominantly unmutated immunoglobulin heavy chain genes, with upregulated tyrosine kinase ZAP-70 expression and elevated ERK-MAPK-mTor signaling, resulting in enhanced proliferation and increased tumor load in lymphoid organs. Reduced function of PKCα leads to an up-regulation of PKCβII expression, which is also associated with a poor prognostic subset of human chronic lymphocytic leukemia samples. Treatment of chronic lymphocytic leukemia-like cells with the selective PKCβ inhibitor enzastaurin caused cell cycle arrest and apoptosis both in vitro and in vivo, and a reduction in the leukemic burden in vivo. These results demonstrate the importance of PKCβII in chronic lymphocytic leukemia-like disease progression and suggest a role for PKCα subversion in creating permissive conditions for leukemogenesis.
Copyright© Ferrata Storti Foundation.
Figures




References
-
- Dighiero G. CLL Biology and prognosis. Hematology Am Soc Hematol Educ Program. 2005:278–84. - PubMed
-
- Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82(6):1820–1828. - PubMed
-
- Ghia P, Chiorazzi N, Stamatopoulos K. Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J Intern Med. 2008;264(6):549–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

