Animals deficient in C2Orf71, an autosomal recessive retinitis pigmentosa-associated locus, develop severe early-onset retinal degeneration
- PMID: 25616964
- PMCID: PMC4383867
- DOI: 10.1093/hmg/ddv025
Animals deficient in C2Orf71, an autosomal recessive retinitis pigmentosa-associated locus, develop severe early-onset retinal degeneration
Abstract
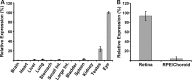
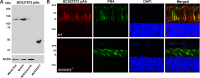
Genetic mapping was recently used to identify the underlying cause for a previously uncharacterized cohort of autosomal recessive retinitis pigmentosa cases. Genetic mapping of affected individuals resulted in the identification of an uncharacterized gene, C2Orf71, as the causative locus. However, initial homology searches failed to reveal similarities to any previously characterized protein or domain. To address this issue, we characterized the mouse homolog, BC027072. Immunohistochemistry with a custom polyclonal antibody showed staining localized to the inner segments (IS) of photoreceptor cells, as well as the outer segments (OS) of cone cells. A knockout mouse line (BC(-/-)) was generated and demonstrated that loss of this gene results in a severe, early-onset retinal degeneration. Histology and electron microscopy (EM) revealed disorganized OS as early as 3 weeks with complete loss by 24 weeks of age. EM micrographs displayed packets of cellular material containing OS discs or IS organelles in the OS region and abnormal retinal pigmented epithelium cells. Analyses of retinoids and rhodopsin levels showed <20% in BC(-/-) versus wild-type mice early in development. Electroretinograms demonstrated that affected mice were virtually non-responsive to light by 8 weeks of age. Lastly, RNAseq analysis of ocular gene expression in BC(-/-) mice revealed clues to the causes of the progressive retinal degenerations. Although its function remains unknown, this protein appears essential for normal OS development/maintenance and vision in humans and mice. RNAseq data are available in the GEO database under accession: GSE63810.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures







References
-
- Hildebrand G.D., Fielder A.R. (2011) Anatomy and physiology of the retina In Reynolds J.D., Olitsky S.E. (eds), Pediatric Retina. Springer, Berlin, Germany, pp. 39–65.
-
- Ridge K.D., Abdulaev N.G., Sousa M., Palczewski K. (2003) Phototransduction: crystal clear. Trends Biochem. Sci., 28, 479–487. - PubMed
-
- Sahel J., Bonnel S., Mrejen S., Paques M. (2010) Retinitis pigmentosa and other dystrophies In Coscas G., CunhaVaz J., Loewenstein A., Soubrane G. (eds), Macular Edema: A Practical Approach. Karger, Postfach, Basel, Switzerland, Vol. 47, pp. 160–167.
-
- Anasagasti A., Irigoyen C., Barandika O., de Munain A.L., Ruiz-Ederra J. (2012) Current mutation discovery approaches in retinitis pigmentosa. Vision Res., 75, 117–129. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

