Role of SM22 in the differential regulation of phasic vs. tonic smooth muscle
- PMID: 25617350
- PMCID: PMC4385893
- DOI: 10.1152/ajpgi.00360.2014
Role of SM22 in the differential regulation of phasic vs. tonic smooth muscle
Abstract
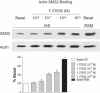
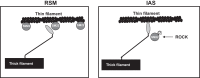
Preliminary proteomics studies between tonic vs. phasic smooth muscles identified three distinct protein spots identified to be those of transgelin (SM22). The latter was found to be distinctly downregulated in the internal anal sphincter (IAS) vs. rectal smooth muscle (RSM) SMC. The major focus of the present studies was to examine the differential molecular control mechanisms by SM22 in the functionality of truly tonic smooth muscle of the IAS vs. the adjoining phasic smooth muscle of the RSM. We monitored SMC lengths before and after incubation with pFLAG-SM22 (for SM22 overexpression), and SM22 small-interfering RNA. pFLAG-SM22 caused concentration-dependent and significantly greater relaxation in the IAS vs. the RSM SMCs. Conversely, temporary silencing of SM22 caused contraction in both types of the SMCs. Further studies revealed a significant reverse relationship between the levels of SM22 phosphorylation and the amount of SM22-actin binding in the IAS and RSM SMC. Data showed higher phospho-SM22 levels and decreased SM22-actin binding in the IAS, and reverse to be the case in the RSM SMCs. Experiments determining the mechanism for SM22 phosphorylation in these smooth muscles revealed that Y-27632 (Rho kinase inhibitor) but not Gö-6850 (protein kinase C inhibitor) caused concentration-dependent decreased phosphorylation of SM22. We speculate that SM22 plays an important role in the regulation of basal tone via Rho kinase-induced phosphorylation of SM22.
Keywords: actin-myosin binding; proteomics; transgelin-actin binding.
Copyright © 2015 the American Physiological Society.
Figures






Similar articles
-
2D DIGE Does Not Reveal all: A Scotopic Report Suggests Differential Expression of a Single "Calponin Family Member" Protein for Tetany of Sphincters!Front Med (Lausanne). 2015 Jun 18;2:42. doi: 10.3389/fmed.2015.00042. eCollection 2015. Front Med (Lausanne). 2015. PMID: 26151053 Free PMC article. Review.
-
Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK.Am J Physiol Gastrointest Liver Physiol. 2007 Jun;292(6):G1747-56. doi: 10.1152/ajpgi.00438.2006. Epub 2007 Mar 22. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17379756
-
Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle.Am J Physiol Gastrointest Liver Physiol. 2006 Nov;291(5):G830-7. doi: 10.1152/ajpgi.00130.2006. Epub 2006 Jun 8. Am J Physiol Gastrointest Liver Physiol. 2006. PMID: 16763289
-
Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter.Gastroenterology. 2006 Jul;131(1):108-16. doi: 10.1053/j.gastro.2006.03.043. Gastroenterology. 2006. PMID: 16831595
-
Transgelins, cytoskeletal proteins implicated in different aspects of cancer development.Expert Rev Proteomics. 2014 Apr;11(2):149-65. doi: 10.1586/14789450.2014.860358. Epub 2014 Jan 29. Expert Rev Proteomics. 2014. PMID: 24476357 Review.
Cited by
-
Deletion of SM22α disrupts the structure and function of caveolae and T-tubules in cardiomyocytes, contributing to heart failure.PLoS One. 2022 Jul 18;17(7):e0271578. doi: 10.1371/journal.pone.0271578. eCollection 2022. PLoS One. 2022. PMID: 35849583 Free PMC article.
-
Altered Esophageal Smooth Muscle Phenotype in Achalasia.J Neurogastroenterol Motil. 2024 Apr 30;30(2):166-176. doi: 10.5056/jnm23024. Epub 2023 Aug 2. J Neurogastroenterol Motil. 2024. PMID: 37528076 Free PMC article.
-
2D DIGE Does Not Reveal all: A Scotopic Report Suggests Differential Expression of a Single "Calponin Family Member" Protein for Tetany of Sphincters!Front Med (Lausanne). 2015 Jun 18;2:42. doi: 10.3389/fmed.2015.00042. eCollection 2015. Front Med (Lausanne). 2015. PMID: 26151053 Free PMC article. Review.
-
ORMDL3 expression in ASM regulates hypertrophy, hyperplasia via TPM1 and TPM4, and contractility.JCI Insight. 2021 Apr 8;6(7):e136911. doi: 10.1172/jci.insight.136911. JCI Insight. 2021. PMID: 33661765 Free PMC article.
References
-
- Alban A, David SO, Bjorkesten L, Andersson C, Sloge E, Lewis S, Currie I. A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics 3: 36–44, 2003. - PubMed
-
- Almendral JM, Santaren JF, Parera J, Zerial M, Bravo R. Expression, cloning and cDNA sequence of a fibroblast serum-regulated gene encoding a putative actin-associated protein (p27). Exp Cell Res 181: 518–530, 1989. - PubMed
-
- Biancani P, Sohn UD, Rich HG, Harnett KM, Behar J. Signal transduction pathways in esophageal and lower esophageal sphincter circular muscle. Am J Med Suppl 103: 23S–28S, 1997. - PubMed
-
- Camoretti-Mecado B, Forsythe SM, LeBeau MM, Espinosa R, Vieira JE, Halayko AJ, Willadsen S, Ober C, Evan GA, Thweatt R, Shapiro S, Niu Q, Qin Y, Padrid PA, Solway J. Expression and cytogenetic localization of the human SM22 gene (TAGLN). Genomics 49: 452–457, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

