Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity
- PMID: 25617503
- PMCID: PMC4451470
- DOI: 10.1016/j.jhep.2014.12.034
Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity
Abstract
Background & aims: Bile acids (BAs) are major regulators of hepatic BA and lipid metabolism but their mechanisms of action in non-alcoholic fatty liver disease (NAFLD) are still poorly understood. Here we aimed to explore the molecular and biochemical mechanisms of ursodeoxycholic acid (UDCA) in modulating the cross-talk between liver and visceral white adipose tissue (vWAT) regarding BA and cholesterol metabolism and fatty acid/lipid partitioning in morbidly obese NAFLD patients.
Methods: In this randomized controlled pharmacodynamic study, we analyzed serum, liver and vWAT samples from 40 well-matched morbidly obese patients receiving UDCA (20 mg/kg/day) or no treatment three weeks prior to bariatric surgery.
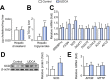
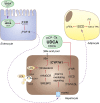
Results: Short term UDCA administration stimulated BA synthesis by reducing circulating fibroblast growth factor 19 and farnesoid X receptor (FXR) activation, resulting in cholesterol 7α-hydroxylase induction mirrored by elevated C4 and 7α-hydroxycholesterol. Enhanced BA formation depleted hepatic and LDL-cholesterol with subsequent activation of the key enzyme of cholesterol synthesis 3-hydroxy-3-methylglutaryl-CoA reductase. Blunted FXR anti-lipogenic effects induced lipogenic stearoyl-CoA desaturase (SCD) in the liver, thereby increasing hepatic triglyceride content. In addition, induced SCD activity in vWAT shifted vWAT lipid metabolism towards generation of less toxic and more lipogenic monounsaturated fatty acids such as oleic acid.
Conclusion: These data demonstrate that by exerting FXR-antagonistic effects, UDCA treatment in NAFLD patients strongly impacts on cholesterol and BA synthesis and induces neutral lipid accumulation in both liver and vWAT.
Keywords: 3-hydroxy-3-methylglutaryl-CoA reductase; FGF19; Lipogenesis; Non-alcoholic fatty liver disease; Stearoyl-CoA desaturase.
Copyright © 2015 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures



Comment in
-
Inhibition of farnesoid X receptor signaling shows beneficial effects in human obesity.J Hepatol. 2015 Jun;62(6):1234-6. doi: 10.1016/j.jhep.2015.02.043. Epub 2015 Mar 5. J Hepatol. 2015. PMID: 25747705 Free PMC article. No abstract available.
Similar articles
-
Protective effects of farnesoid X receptor (FXR) on hepatic lipid accumulation are mediated by hepatic FXR and independent of intestinal FGF15 signal.Liver Int. 2015 Apr;35(4):1133-1144. doi: 10.1111/liv.12456. Epub 2014 Feb 7. Liver Int. 2015. PMID: 25156247 Free PMC article.
-
Hyperoside attenuates non-alcoholic fatty liver disease in rats via cholesterol metabolism and bile acid metabolism.J Adv Res. 2021 Jun 8;34:109-122. doi: 10.1016/j.jare.2021.06.001. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024184 Free PMC article.
-
Effects of therapeutically approved individual bile acids on the development of metabolic dysfunction-associated steatohepatitis a low bile acid mouse model.Toxicol Sci. 2024 Dec 1;202(2):179-195. doi: 10.1093/toxsci/kfae110. Toxicol Sci. 2024. PMID: 39302723 Free PMC article.
-
Bile acids as regulators of hepatic lipid and glucose metabolism.Dig Dis. 2010;28(1):220-4. doi: 10.1159/000282091. Epub 2010 May 7. Dig Dis. 2010. PMID: 20460915 Review.
-
Recent Advances in the Digestive, Metabolic and Therapeutic Effects of Farnesoid X Receptor and Fibroblast Growth Factor 19: From Cholesterol to Bile Acid Signaling.Nutrients. 2022 Nov 22;14(23):4950. doi: 10.3390/nu14234950. Nutrients. 2022. PMID: 36500979 Free PMC article. Review.
Cited by
-
Nonalcoholic Fatty Liver Disease (NAFLD). Mitochondria as Players and Targets of Therapies?Int J Mol Sci. 2021 May 20;22(10):5375. doi: 10.3390/ijms22105375. Int J Mol Sci. 2021. PMID: 34065331 Free PMC article. Review.
-
Ursodeoxycholic Acid Treatment Restores Gut Microbiota and Alleviates Liver Inflammation in Non-Alcoholic Steatohepatitic Mouse Model.Front Pharmacol. 2021 Dec 6;12:788558. doi: 10.3389/fphar.2021.788558. eCollection 2021. Front Pharmacol. 2021. PMID: 34938193 Free PMC article.
-
Versatile Triad Alliance: Bile Acid, Taurine and Microbiota.Cells. 2022 Jul 29;11(15):2337. doi: 10.3390/cells11152337. Cells. 2022. PMID: 35954180 Free PMC article. Review.
-
Farnesoid X receptor and fibroblast growth factor 15/19 as pharmacological targets.Liver Res. 2021 Mar 9;5(3):142-150. doi: 10.1016/j.livres.2021.02.002. eCollection 2021 Sep. Liver Res. 2021. PMID: 39957843 Free PMC article. Review.
-
An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease.Gastroenterology. 2016 Nov;151(5):845-859. doi: 10.1053/j.gastro.2016.08.057. Epub 2016 Sep 14. Gastroenterology. 2016. PMID: 27639801 Free PMC article. Review.
References
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. - PubMed
-
- Kalaany N.Y., Mangelsdorf D.J. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Ann Rev Physiol. 2006;68:159–191. - PubMed
-
- Claudel T., Inoue Y., Barbier O., Duran-Sandoval D., Kosykh V., Fruchart J., et al. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

