International STakeholder NETwork (ISTNET): creating a developmental neurotoxicity (DNT) testing road map for regulatory purposes
- PMID: 25618548
- PMCID: PMC4309915
- DOI: 10.1007/s00204-015-1464-2
International STakeholder NETwork (ISTNET): creating a developmental neurotoxicity (DNT) testing road map for regulatory purposes
Abstract
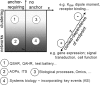
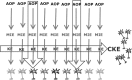
A major problem in developmental neurotoxicity (DNT) risk assessment is the lack of toxicological hazard information for most compounds. Therefore, new approaches are being considered to provide adequate experimental data that allow regulatory decisions. This process requires a matching of regulatory needs on the one hand and the opportunities provided by new test systems and methods on the other hand. Alignment of academically and industrially driven assay development with regulatory needs in the field of DNT is a core mission of the International STakeholder NETwork (ISTNET) in DNT testing. The first meeting of ISTNET was held in Zurich on 23-24 January 2014 in order to explore the concept of adverse outcome pathway (AOP) to practical DNT testing. AOPs were considered promising tools to promote test systems development according to regulatory needs. Moreover, the AOP concept was identified as an important guiding principle to assemble predictive integrated testing strategies (ITSs) for DNT. The recommendations on a road map towards AOP-based DNT testing is considered a stepwise approach, operating initially with incomplete AOPs for compound grouping, and focussing on key events of neurodevelopment. Next steps to be considered in follow-up activities are the use of case studies to further apply the AOP concept in regulatory DNT testing, making use of AOP intersections (common key events) for economic development of screening assays, and addressing the transition from qualitative descriptions to quantitative network modelling.
Figures



References
-
- Ankley GT, Bennett RS, Erickson RJ, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–741. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

