Systemic inflammatory disease resolution following cosmetic silicone breast implant removal
- PMID: 25618880
- PMCID: PMC4307040
- DOI: 10.1136/bcr-2014-207418
Systemic inflammatory disease resolution following cosmetic silicone breast implant removal
Abstract
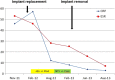
A 37-year-old Caucasian woman presented with subacute, symmetrical inflammatory arthralgia, which was affecting her work. Apart from fatigue, she had no other constitutional symptoms. She had undergone cosmetic bilateral silicone breast implant surgery in 2008. Blood tests revealed erythrocyte sedimentation rate 53 mm/h, weakly positive antinuclear antibodies and IgG cardiolipin antibody, while breast ultrasound revealed a ruptured left silicone implant. The working diagnosis was systemic inflammatory disease of uncertain origin. She decided to have replacement, rather than removal, of her silicone breast implants privately, but her symptoms persisted postoperatively with a new erythema multiforme-like rash despite treatment with methotrexate and moderate dose prednisolone. Following further consultation with a National Health Service breast surgeon, her silicone implants were removed. Within 10 weeks of surgery, all immunomodulatory treatment was discontinued with complete symptom and inflammatory response resolution. This case illustrates that implant silicone can induce clinically significant systemic inflammatory disease and implant removal is essential for disease resolution.
2015 BMJ Publishing Group Ltd.
Figures
Similar articles
-
Intravascular large B-cell lymphoma associated with silicone breast implant, HLA-DRB1*11:01, and HLA-DQB1*03:01 manifesting as macrophage activation syndrome and with severe neurological symptoms: a case report.J Med Case Rep. 2016 Sep 15;10(1):254. doi: 10.1186/s13256-016-0993-5. J Med Case Rep. 2016. PMID: 27634631 Free PMC article.
-
Silicone implant incompatibility syndrome (SIIS) in a 57-year-old woman with unilateral silicone breast implant.BMJ Case Rep. 2017 Jul 24;2017:bcr2016218709. doi: 10.1136/bcr-2016-218709. BMJ Case Rep. 2017. PMID: 28739610 Free PMC article.
-
PIP silicone breast implants: rupture rates based on the explantation of 676 implants in a single surgeon series.J Plast Reconstr Aesthet Surg. 2013 Sep;66(9):1182-7. doi: 10.1016/j.bjps.2013.05.003. Epub 2013 May 30. J Plast Reconstr Aesthet Surg. 2013. PMID: 23725742
-
Massive inflammatory reaction following the removal of a ruptured silicone implant masking the invasive breast cancer - case report and literature review.Pol Przegl Chir. 2016 Jan 1;88(1):41-7. doi: 10.1515/pjs-2016-0026. Pol Przegl Chir. 2016. PMID: 27096774 Review.
-
A Review of the Literature on the Management of Silicone Implant Incompatibility Syndrome.Aesthetic Plast Surg. 2019 Oct;43(5):1145-1149. doi: 10.1007/s00266-019-01407-4. Epub 2019 May 29. Aesthetic Plast Surg. 2019. PMID: 31144006 Review.
Cited by
-
Is explantation of silicone breast implants useful in patients with complaints?Immunol Res. 2017 Feb;65(1):25-36. doi: 10.1007/s12026-016-8813-y. Immunol Res. 2017. PMID: 27412295 Free PMC article. Review.
References
-
- Keogh B. Poly implant prosthese (PIP) breast implants: final report of the expert group. June 2012. https://www.gov.uk/government/uploads/system/uploads/attachment_data/fil...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous