Adjuvant therapy in lymph node-positive vulvar cancer: the AGO-CaRE-1 study
- PMID: 25618900
- PMCID: PMC4356703
- DOI: 10.1093/jnci/dju426
Adjuvant therapy in lymph node-positive vulvar cancer: the AGO-CaRE-1 study
Abstract
Background: Women with node-positive vulvar cancer have a high risk for disease recurrence. Indication criteria for adjuvant radiotherapy are controversial. This study was designed to further understand the role of adjuvant therapy in node-positive disease.
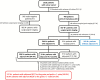
Methods: Patients with primary squamous-cell vulvar cancer treated at 29 gynecologic cancer centers in Germany from 1998 through 2008 were included in this retrospective exploratory multicenter cohort study. Of 1618 documented patients, 1249 had surgical groin staging and known lymph node status and were further analyzed. All statistical tests were two-sided.
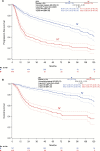
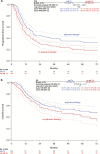
Results: Four hundred forty-seven of 1249 patients (35.8%) had lymph node metastases (N+). The majority of N+ patients had one (172 [38.5%]) or two (102 [22.8%]) positive nodes. The three-year progression-free survival (PFS) rate of N+ patients was 35.2%, and the overall survival (OS) rate 56.2% compared with 75.2% and 90.2% in node-negative patients (N-). Two hundred forty-four (54.6%) N+ patients had adjuvant therapy, of which 183 (40.9%) had radiotherapy directed at the groins (+/-other fields). Three-year PFS and OS rates in these patients were better compared with N+ patients without adjuvant treatment (PFS: 39.6% vs 25.9%, hazard ratio [HR] = 0.67, 95% confidence interval [CI[= 0.51 to 0.88, P = .004; OS: 57.7% vs 51.4%, HR = 0.79, 95% CI = 0.56 to 1.11, P = .17). This effect was statistically significant in multivariable analysis adjusted for age, Eastern Cooperative Oncology Group, Union internationale contre le cancer stage, grade, invasion depth, and number of positive nodes (PFS: HR = 0.58, 95% CI = 0.43 to 0.78, P < .001; OS: HR = 0.63, 95% CI = 0.43 to 0.91, P = .01).
Conclusion: This large multicenter study in vulvar cancer observed that adjuvant radiotherapy was associated with improved prognosis in node-positive patients and will hopefully help to overcome concerns regarding adjuvant treatment. However, outcome after adjuvant radiotherapy remains poor compared with node-negative patients. Adjuvant chemoradiation could be a possible strategy to improve therapy because it is superior to radiotherapy alone in other squamous cell carcinomas.
© The Author 2014. Published by Oxford University Press.
Figures

References
-
- Beller U, Quinn MA, Benedet JL, et al. Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S7–S27. - PubMed
-
- Hampl M, Deckers-Figiel S, Hampl JA, Rein D, Bender HG. New aspects of vulvar cancer: changes in localization and age of onset. Gynecol Oncol. 2008;109(3):340–345. - PubMed
-
- Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018–1022. - PubMed
-
- Gadducci A, Cionini L, Romanini A, Fanucchi A, AR G. Old and new perspectives in the management of high-risk, locally advanced or recurrent, and metastatic vulvar cancer. Crit Rev Oncol/Hematol. 2006;60(3):227–241. - PubMed
-
- Woelber L, Mahner S, Voelker K, et al. Clinicopathological prognostic factors and patterns of recurrence in vulvar cancer. Anticancer Res. 2009;29(2):545–552. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

