Citrate modulates lipopolysaccharide-induced monocyte inflammatory responses
- PMID: 25619261
- PMCID: PMC4449780
- DOI: 10.1111/cei.12591
Citrate modulates lipopolysaccharide-induced monocyte inflammatory responses
Abstract
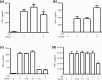
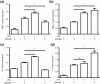
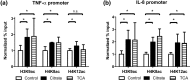
Citrate, a central component of cellular metabolism, is a widely used anti-coagulant due to its ability to chelate calcium. Adenosine triphosphate (ATP)-citrate lyase, which metabolizes citrate, has been shown to be essential for inflammation, but the ability of exogenous citrate to impact inflammatory signalling cascades remains largely unknown. We hypothesized that citrate would modulate inflammatory responses as both a cellular metabolite and calcium chelator, and tested this hypothesis by determining how clinically relevant levels of citrate modulate monocyte proinflammatory responses to lipopolysaccharide (LPS) in a human acute monocytic leukaemia cell line (THP-1). In normal medium (0.4 mM calcium), citrate inhibited LPS-induced tumour necrosis factor (TNF)-α and interleukin (IL)-8 transcripts, whereas in medium supplemented with calcium (1.4 mM), TNF-α and IL-8 levels increased and appeared independent of calcium chelation. Using an IL-8-luciferase plasmid construct, the same increased response was observed in the activation of the IL-8 promoter region, suggesting transcriptional regulation. Tricarballylic acid, an inhibitor of ATP-citrate lyase, blocked the ability of citrate to augment TNF-α, linking citrate's augmentation effect with its metabolism by ATP-citrate lyase. In the presence of citrate, increased histone acetylation was observed in the TNF-α and IL-8 promoter regions of THP-1 cells. We observed that citrate can both augment and inhibit proinflammatory cytokine production via modulation of inflammatory gene transactivation. These findings suggest that citrate anti-coagulation may alter immune function through complex interactions with the inflammatory response.
Keywords: citrate; epigenetics; inflammation; monocytes.
© 2015 British Society for Immunology.
Figures



References
-
- Kao GS, Kim HT, Daley H, et al. Validation of short-term handling and storage conditions for marrow and peripheral blood stem cell products. Transfusion. 2011;51:137–47. - PubMed
-
- Tolwani A, Wille KM. Advances in continuous renal replacement therapy: citrate anticoagulation update. Blood Purif. 2012;34:88–93. - PubMed
-
- Engstad CS, Gutteberg TJ, Osterud B. Modulation of blood cell activation by four commonly used anticoagulants. Thromb Haemost. 1997;77:690–6. - PubMed
-
- Chadha V, Garg U, Warady BA, Alon US. Citrate clearance in children receiving continuous venovenous renal replacement therapy. Pediatr Nephrol. 2002;17:819–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

