Safety, tolerability, and efficacy of azilsartan medoxomil with or without chlorthalidone during and after 8 months of treatment for hypertension
- PMID: 25619410
- PMCID: PMC5024056
- DOI: 10.1111/jch.12474
Safety, tolerability, and efficacy of azilsartan medoxomil with or without chlorthalidone during and after 8 months of treatment for hypertension
Abstract
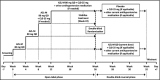
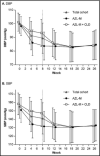
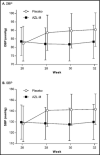
A phase 3, 26-week, open-label, titrate-to-target study (n=418) assessed the safety of azilsartan medoxomil (AZL-M) alone and with chlorthalidone (CLD), followed by a 6-week, double-blind, placebo-controlled reversal phase with change in clinic diastolic blood pressure (DBP) as the primary endpoint. Target blood pressure (BP) was <140/90 mm Hg (<130/80 mm Hg with diabetes/chronic kidney disease). AZL-M was initiated at 40 mg once a day (QD), force-titrated to 80 mg at week 4. CLD 25 mg QD could be added (weeks 8-22), if required, to reach target, followed by additional antihypertensives from week 12. At the end of the open-label phase, mean change in systolic BP (SBP)/DBP from baseline was -23/-16 mm Hg. The most common adverse events, irrespective of treatment, were dizziness (8.9%) and headache (7.2%). Serious AEs were reported in eight patients (1.9%). Consecutive creatinine elevations ≥50% with values exceeding the upper limit of normal (ULN) were reported in nine (2.2%) patients. All returned to below the 50% threshold; most also returned to below the ULN after drug discontinuation. Mean DBP was maintained through the reversal phase in patients receiving AZL-M, but increased with placebo (difference: -7.8 mm Hg, 95% confidence interval, -9.8 to -5.8; P<.001). AZL-M alone or with CLD showed good long-term safety and stable BP improvements in a titrate-to-target approach. BP improvements caused by AZL-M therapy were safely reversible upon AZL-M withdrawal.
Trial registration: ClinicalTrials.gov NCT00696384.
© 2015 Wiley Periodicals, Inc.
Figures
References
-
- Arbor Pharmaceuticals, LLC. Edarbi (azilsartan medoxomil) Tablets. U.S. Prescribing Information. Arbor Pharmaceuticals, LLC, Atlanta, GA, USA. Jul 2004. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200796s006lbl.pdf. Accessed October 13, 2014.
-
- Takeda Pharma A/S. Edarbi (azilsartan medoxomil) Tablets. Summary of Product Characteristics . Takeda Pharma A/S, Taastrup, Denmark. Oct 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Infor.... Accessed October 13, 2014.
-
- Zaiken K, Cheng JW. Azilsartan medoxomil: a new angiotensin receptor blocker. Clin Ther. 2011;33:1577–1589. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

