Persistent DNA damage-induced premature senescence alters the functional features of human bone marrow mesenchymal stem cells
- PMID: 25619736
- PMCID: PMC4395188
- DOI: 10.1111/jcmm.12387
Persistent DNA damage-induced premature senescence alters the functional features of human bone marrow mesenchymal stem cells
Abstract
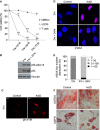
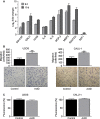
Human mesenchymal stem cells (hMSCs) are adult multipotent stem cells located in various tissues, including the bone marrow. In contrast to terminally differentiated somatic cells, adult stem cells must persist and function throughout life to ensure tissue homeostasis and repair. For this reason, they must be equipped with DNA damage responses able to maintain genomic integrity while ensuring their lifelong persistence. Evaluation of hMSC response to genotoxic insults is of great interest considering both their therapeutic potential and their physiological functions. This study aimed to investigate the response of human bone marrow MSCs to the genotoxic agent Actinomycin D (ActD), a well-known anti-tumour drug. We report that hMSCs react by undergoing premature senescence driven by a persistent DNA damage response activation, as hallmarked by inhibition of DNA synthesis, p21 and p16 protein expression, marked Senescent Associated β-galactosidase activity and enlarged γH2AX foci co-localizing with 53BP1 protein. Senescent hMSCs overexpress several senescence-associated secretory phenotype (SASP) genes and promote motility of lung tumour and osteosarcoma cell lines in vitro. Our findings disclose a multifaceted consequence of ActD treatment on hMSCs that on the one hand helps to preserve this stem cell pool and prevents damaged cells from undergoing neoplastic transformation, and on the other hand alters their functional effects on the surrounding tissue microenvironment in a way that might worsen their tumour-promoting behaviour.
Keywords: DNA damage; actinomycin D; mesenchymal stem cell; senescence-associated secretory phenotype; stress-induced premature senescence.
© 2015 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. - PubMed
-
- Frenette PS, Pinho S, Lucas D, et al. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285–316. - PubMed
-
- Nery AA, Nascimento IC, Glaser T, et al. Human mesenchymal stem cells: from immunophenotyping by flow cytometry to clinical applications. Cytometry A. 2013;83:48–61. - PubMed
-
- Sperka T, Wang J, Rudolph KL. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13:579–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

