Ameliorating treatment-refractory depression with intranasal ketamine: potential NMDA receptor actions in the pain circuitry representing mental anguish
- PMID: 25619798
- PMCID: PMC4515405
- DOI: 10.1017/S1092852914000686
Ameliorating treatment-refractory depression with intranasal ketamine: potential NMDA receptor actions in the pain circuitry representing mental anguish
Abstract
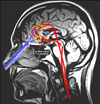
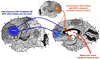
This article reviews the antidepressant actions of ketamine, an N-methyl-D-aspartame glutamate receptor (NMDAR) antagonist, and offers a potential neural mechanism for intranasal ketamine's ultra-rapid actions based on the key role of NMDAR in the nonhuman primate prefrontal cortex (PFC). Although intravenous ketamine infusions can lift mood within hours, the current review describes how intranasal ketamine administration can have ultra-rapid antidepressant effects, beginning within minutes (5-40 minutes) and lasting hours, but with repeated treatments needed for sustained antidepressant actions. Research in rodents suggests that increased synaptogenesis in PFC may contribute to the prolonged benefit of ketamine administration, beginning hours after administration. However, these data cannot explain the relief that occurs within minutes of intranasal ketamine delivery. We hypothesize that the ultra-rapid effects of intranasal administration in humans may be due to ketamine blocking the NMDAR circuits that generate the emotional representations of pain (eg, Brodmann Areas 24 and 25, insular cortex), cortical areas that can be overactive in depression and which sit above the nasal epithelium. In contrast, NMDAR blockade in the dorsolateral PFC following systemic administration of ketamine may contribute to cognitive deficits. This novel view may help to explain how intravenous ketamine can treat the symptoms of depression yet worsen the symptoms of schizophrenia.
Keywords: Antidepressant; area 25; glutamate; prefrontal cortex; rTMS.
Figures

References
-
- Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36(2):186–197. - PubMed
-
- Andolfatto G, Willman E, Joo D, et al. Intranasal ketamine for analgesia in the emergency department: a prospective observational series. Acad Emerg Med. 2013;20(10):1050–1054. - PubMed
-
- McCarty EC, Mencio GA, Walker LA, Green NE. Ketamine sedation for the reduction of children’s fractures in the emergency department. J Bone Joint Surg Am. 2000;82-A(7):912–918. - PubMed
-
- Moretti RJ, Hassan SZ, Goodman LI, Meltzer HY. Comparison of ketamine and thiopental in healthy volunteers: effects on mental status, mood, and personality. Anesth Analg. 1984;63(12):1087–1096. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

