Erythropoietin in stroke therapy: friend or foe
- PMID: 25620101
- PMCID: PMC4497368
- DOI: 10.2174/0929867322666150114152134
Erythropoietin in stroke therapy: friend or foe
Abstract
Recombinant human erythropoietin (rhEPO), over the past decade, was hailed as an auspicious therapeutic strategy for various types of brain injuries. The promising results from experiments conducted in animal models of stroke led to a hurried clinical trial that was swiftly aborted in Phase II. The multiple neuroprotective modalities of rhEPO failed to translate smoothly to human adult ischemic brain injury and provided limited aid to neonates. In light of the antithetical results, several questions were raised as to why and how this clinical trial failed. There was bolstering evidence from the preliminary studies that pointed to a bright future. Therefore, the objective of this review is to address these questions by discussing the signaling pathways of rhEPO that are reported to mediate the neuroprotective effect in various animal models of brain injury. Major biomedical bibliographical databases (MEDLINE, ISI, PubMed, and Cochrane Library) were searched with the use of keywords such as erythropoietin, stroke, neonatal hypoxia ischemia, intracerebral hemorrhage, etc. This article will discuss the confounding factors that influence the efficacy of rhEPO treatment hence challenging its clinical translatability. Lastly, rhEPO may still be a promising therapeutic candidate for neonates in spite of its shortcoming in clinical trial if caution is taken with the dose and duration of its administration.
Conflict of interest statement
The authors confirm that this article content has no conflict of interest.
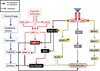
Figures
References
-
- Alafaci C, Salpietro F, Grasso G, Sfacteria A, Passalacqua M, Morabito A, Tripodo E, Calapai G, Buemi M, Tomasello F. Effect of recombinant human erythropoietin on cerebral ischemia following experimental subarachnoid hemorrhage. Eur. J. Pharmacol. 2000;406(2):219–225. - PubMed
-
- Grasso G. Neuroprotective effect of recombinant human erythropoietin in experimental subarachnoid hemorrhage. J. Neurosurg Sci. 2001;45(1):7–14. - PubMed
-
- Grasso G, Buemi M, Alafaci C, Sfacteria A, Passalacqua M, Sturiale A, Calapai G, De Vico G, Piedimonte G, Salpietro FM, et al. Beneficial effects of systemic administration of recombinant human erythropoietin in rabbits subjected to subarachnoid hemorrhage. Proc. Natl. Acad. Sci. U S A. 2002;99(8):5627–5631. - PMC - PubMed
-
- Grasso G. An overview of new pharmacological treatments for cerebrovascular dysfunction after experimental subarachnoid hemorrhage. Brain Res. Brain Res. Rev. 2004;44(1):49–63. - PubMed
-
- Lee ST, Chu K, Sinn DI, Jung KH, Kim EH, Kim SJ, Kim JM, Ko SY, Kim M, Roh JK. Erythropoietin reduces perihematomal inflammation and cell death with eNOS and STAT3 activations in experimental intracerebral hemorrhage. J. Neurochem. 2006;96(6):1728–1739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

