Loss of hepatic chaperone-mediated autophagy accelerates proteostasis failure in aging
- PMID: 25620427
- PMCID: PMC4364837
- DOI: 10.1111/acel.12310
Loss of hepatic chaperone-mediated autophagy accelerates proteostasis failure in aging
Abstract
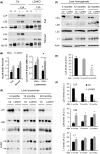
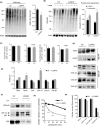
Chaperone-mediated autophagy (CMA), a cellular process that contributes to protein quality control through targeting of a subset of cytosolic proteins to lysosomes for degradation, undergoes a functional decline with age. We have used a mouse model with liver-specific defective CMA to identify changes in proteostasis attributable to reduced CMA activity in this organ with age. We have found that other proteolytic systems compensate for CMA loss in young mice which helps to preserve proteostasis. However, these compensatory responses are not sufficient for protection against proteotoxicity induced by stress (oxidative stress, lipid challenges) or associated with aging. Livers from old mice with CMA blockage exhibit altered protein homeostasis, enhanced susceptibility to oxidative stress and hepatic dysfunction manifested by a diminished ability to metabolize drugs, and a worsening of the metabolic dysregulation identified in young mice. Our study reveals that while the regulatory function of CMA cannot be compensated for in young organisms, its contribution to protein homeostasis can be handled by other proteolytic systems. However, the decline in the compensatory ability identified with age explains the more severe consequences of CMA impairment in older organisms and the contribution of CMA malfunction to the gradual decline in proteostasis and stress resistance observed during aging.
Keywords: autophagy; lysosomal protein degradation; macroautophagy; oxidative stress; protein aggregation; proteotoxicity; ubiquitin-proteasome system.
© 2015 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures





Similar articles
-
Chaperone mediated autophagy in aging: Starve to prosper.Ageing Res Rev. 2016 Dec;32:13-21. doi: 10.1016/j.arr.2016.07.001. Epub 2016 Jul 30. Ageing Res Rev. 2016. PMID: 27484893 Review.
-
Early cellular changes after blockage of chaperone-mediated autophagy.Autophagy. 2008 May;4(4):442-56. doi: 10.4161/auto.5654. Epub 2008 Jan 30. Autophagy. 2008. PMID: 18253088
-
Chaperone-mediated autophagy: a gatekeeper of neuronal proteostasis.Autophagy. 2021 Aug;17(8):2040-2042. doi: 10.1080/15548627.2021.1935007. Epub 2021 Jun 10. Autophagy. 2021. PMID: 34110247 Free PMC article. Review.
-
Pathophysiology of chaperone-mediated autophagy.Int J Biochem Cell Biol. 2004 Dec;36(12):2420-34. doi: 10.1016/j.biocel.2004.04.010. Int J Biochem Cell Biol. 2004. PMID: 15325582 Review.
-
Connecting chaperone-mediated autophagy dysfunction to cellular senescence.Ageing Res Rev. 2018 Jan;41:34-41. doi: 10.1016/j.arr.2017.11.001. Epub 2017 Nov 4. Ageing Res Rev. 2018. PMID: 29113832 Review.
Cited by
-
The lysosomal proteome of senescent cells contributes to the senescence secretome.Aging Cell. 2022 Oct;21(10):e13707. doi: 10.1111/acel.13707. Epub 2022 Sep 10. Aging Cell. 2022. PMID: 36087066 Free PMC article.
-
The Role of Chaperone-Mediated Autophagy in Tissue Homeostasis and Disease Pathogenesis.Biomedicines. 2024 Jan 23;12(2):257. doi: 10.3390/biomedicines12020257. Biomedicines. 2024. PMID: 38397859 Free PMC article. Review.
-
Chaperone-mediated autophagy and disease: Implications for cancer and neurodegeneration.Mol Aspects Med. 2021 Dec;82:101025. doi: 10.1016/j.mam.2021.101025. Epub 2021 Oct 7. Mol Aspects Med. 2021. PMID: 34629183 Free PMC article.
-
Modulating Chaperone-Mediated Autophagy and Its Clinical Applications in Cancer.Cells. 2022 Aug 17;11(16):2562. doi: 10.3390/cells11162562. Cells. 2022. PMID: 36010638 Free PMC article. Review.
-
LAMP2A, LAMP2B and LAMP2C: similar structures, divergent roles.Autophagy. 2023 Nov;19(11):2837-2852. doi: 10.1080/15548627.2023.2235196. Epub 2023 Jul 21. Autophagy. 2023. PMID: 37469132 Free PMC article. Review.
References
-
- Aniento F, Papavassiliou AG, Knecht E, Roche E. Selective uptake and degradation of c-Fos and v-Fos by rat liver lysosomes. FEBS Lett. 1996;390:47–52. - PubMed
-
- Chiang H, Terlecky S, Plant C, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG021904/AG/NIA NIH HHS/United States
- P30 DK041296/DK/NIDDK NIH HHS/United States
- AG031782/AG/NIA NIH HHS/United States
- R01 AG045223/AG/NIA NIH HHS/United States
- P30 AG038072/AG/NIA NIH HHS/United States
- F30AG046109/AG/NIA NIH HHS/United States
- P01 AG031782/AG/NIA NIH HHS/United States
- R01 DK091466/DK/NIDDK NIH HHS/United States
- R37 AG021904/AG/NIA NIH HHS/United States
- R01 AI137198/AI/NIAID NIH HHS/United States
- AG038072/AG/NIA NIH HHS/United States
- R01 DK098408/DK/NIDDK NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- F30 AG046109/AG/NIA NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- T32-GM007288/GM/NIGMS NIH HHS/United States
- AG21904/AG/NIA NIH HHS/United States
- DK098408/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

