Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia
- PMID: 25620979
- PMCID: PMC4288046
- DOI: 10.3389/fgene.2014.00456
Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia
Abstract
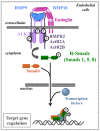
Rendu-Osler-Weber syndrome, also known as hereditary hemorrhagic telangiectasia (HHT), is an autosomal dominant vascular disorder. Three genes are causally related to HHT: the ENG gene encoding endoglin, a co-receptor of the TGFβ family (HHT1), the ACVRL1 gene encoding ALK1 (activin receptor-like kinase 1), a type I receptor of the TGFβ family (HHT2), and the SMAD4 gene, encoding a transcription factor critical for this signaling pathway. Bone morphogenetic proteins (BMPs) are growth factors of the TGFβ family. Among them, BMP9 and BMP10 have been shown to bind directly with high affinity to ALK1 and endoglin, and BMP9 mutations have recently been linked to a vascular anomaly syndrome that has phenotypic overlap with HHT. BMP9 and BMP10 are both circulating cytokines in blood, and the current working model is that BMP9 and BMP10 maintain a quiescent endothelial state that is dependent on the level of ALK1/endoglin activation in endothelial cells. In accordance with this model, to explain the etiology of HHT we hypothesize that a deficient BMP9/BMP10/ALK1/endoglin pathway may lead to re-activation of angiogenesis or a greater sensitivity to an angiogenic stimulus. Resulting endothelial hyperproliferation and hypermigration may lead to vasodilatation and generation of an arteriovenous malformation (AVM). HHT would thus result from a defect in the angiogenic balance. This review will focus on the emerging role played by BMP9 and BMP10 in the development of this disease and the therapeutic approaches that this opens.
Keywords: ALK1; BMP10; BMP9; cell signaling; endoglin; hereditary hemorrhagic telangiectasia.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

