Epigenetic therapy of cancer stem and progenitor cells by targeting DNA methylation machineries
- PMID: 25621113
- PMCID: PMC4300924
- DOI: 10.4252/wjsc.v7.i1.137
Epigenetic therapy of cancer stem and progenitor cells by targeting DNA methylation machineries
Abstract
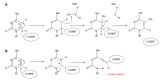
Recent advances in stem cell biology have shed light on how normal stem and progenitor cells can evolve to acquire malignant characteristics during tumorigenesis. The cancer counterparts of normal stem and progenitor cells might be occurred through alterations of stem cell fates including an increase in self-renewal capability and a decrease in differentiation and/or apoptosis. This oncogenic evolution of cancer stem and progenitor cells, which often associates with aggressive phenotypes of the tumorigenic cells, is controlled in part by dysregulated epigenetic mechanisms including aberrant DNA methylation leading to abnormal epigenetic memory. Epigenetic therapy by targeting DNA methyltransferases (DNMT) 1, DNMT3A and DNMT3B via 5-Azacytidine (Aza) and 5-Aza-2'-deoxycytidine (Aza-dC) has proved to be successful toward treatment of hematologic neoplasms especially for patients with myelodysplastic syndrome. In this review, I summarize the current knowledge of mechanisms underlying the inhibition of DNA methylation by Aza and Aza-dC, and of their apoptotic- and differentiation-inducing effects on cancer stem and progenitor cells in leukemia, medulloblastoma, glioblastoma, neuroblastoma, prostate cancer, pancreatic cancer and testicular germ cell tumors. Since cancer stem and progenitor cells are implicated in cancer aggressiveness such as tumor formation, progression, metastasis and recurrence, I propose that effective therapeutic strategies might be achieved through eradication of cancer stem and progenitor cells by targeting the DNA methylation machineries to interfere their "malignant memory".
Keywords: Aza-cytidine; Aza-deoxycytidine; Cancer stem and progenitor cells; DNA methylation; Epigenetic therapy.
Figures

References
-
- Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. - PubMed
-
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Woll PS, Kjällquist U, Chowdhury O, Doolittle H, Wedge DC, Thongjuea S, Erlandsson R, Ngara M, Anderson K, Deng Q, et al. Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell. 2014;25:794–808. - PubMed
-
- Liu YP, Yang CJ, Huang MS, Yeh CT, Wu AT, Lee YC, Lai TC, Lee CH, Hsiao YW, Lu J, et al. Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating Notch signaling. Cancer Res. 2013;73:406–416. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

