Biosynthesis of fosfazinomycin is a convergent process
- PMID: 25621145
- PMCID: PMC4303578
- DOI: 10.1039/C4SC03095H
Biosynthesis of fosfazinomycin is a convergent process
Abstract
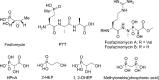
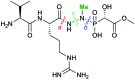
Fosfazinomycin A is a phosphonate natural product in which the C-terminal carboxylate of a Val-Arg dipeptide is connected to methyl 2-hydroxy-2-phosphono-acetate (Me-HPnA) via a unique hydrazide linkage. We report here that Me-HPnA is generated from phosphonoacetaldehyde (PnAA) in three biosynthetic steps through the combined action of an O-methyltransferase (FzmB) and an α-ketoglutarate (α-KG) dependent non-heme iron dioxygenase (FzmG). Unexpectedly, the latter enzyme is involved in two different steps, oxidation of the PnAA to phosphonoacetic acid as well as hydroxylation of methyl 2-phosphonoacetate. The N-methyltransferase (FzmH) was able to methylate Arg-NHNH2 (3) to give Arg-NHNHMe (4), constituting the second segment of the fosfazinomycin molecule. Methylation of other putative intermediates such as desmethyl fosfazinomycin B was not observed. Collectively, our current data support a convergent biosynthetic pathway to fosfazinomycin.
Figures




Similar articles
-
Glutamic acid is a carrier for hydrazine during the biosyntheses of fosfazinomycin and kinamycin.Nat Commun. 2018 Sep 11;9(1):3687. doi: 10.1038/s41467-018-06083-7. Nat Commun. 2018. PMID: 30206228 Free PMC article.
-
New Insights into the Biosynthesis of Fosfazinomycin.Chem Sci. 2016;7(8):5219-5223. doi: 10.1039/C6SC01389A. Epub 2016 May 6. Chem Sci. 2016. PMID: 28070267 Free PMC article.
-
The first direct characterization of a high-valent iron intermediate in the reaction of an alpha-ketoglutarate-dependent dioxygenase: a high-spin FeIV complex in taurine/alpha-ketoglutarate dioxygenase (TauD) from Escherichia coli.Biochemistry. 2003 Jun 24;42(24):7497-508. doi: 10.1021/bi030011f. Biochemistry. 2003. PMID: 12809506
-
Biosynthesis of Lincosamide Antibiotics: Reactions Associated with Degradation and Detoxification Pathways Play a Constructive Role.Acc Chem Res. 2018 Jun 19;51(6):1496-1506. doi: 10.1021/acs.accounts.8b00135. Epub 2018 May 24. Acc Chem Res. 2018. PMID: 29792672 Review.
-
[Advances in hydroxylation of hydrophobic amino acid].Sheng Wu Gong Cheng Xue Bao. 2018 Jul 25;34(7):1046-1056. doi: 10.13345/j.cjb.170509. Sheng Wu Gong Cheng Xue Bao. 2018. PMID: 30058304 Review. Chinese.
Cited by
-
Amazing Diversity in Biochemical Roles of Fe(II)/2-Oxoglutarate Oxygenases.Trends Biochem Sci. 2018 Jul;43(7):517-532. doi: 10.1016/j.tibs.2018.04.002. Epub 2018 Apr 27. Trends Biochem Sci. 2018. PMID: 29709390 Free PMC article. Review.
-
Convergent and divergent biosynthetic strategies towards phosphonic acid natural products.Curr Opin Chem Biol. 2022 Dec;71:102214. doi: 10.1016/j.cbpa.2022.102214. Epub 2022 Oct 3. Curr Opin Chem Biol. 2022. PMID: 36202046 Free PMC article. Review.
-
An inventory of early branch points in microbial phosphonate biosynthesis.Microb Genom. 2022 Feb;8(2):000781. doi: 10.1099/mgen.0.000781. Microb Genom. 2022. PMID: 35188456 Free PMC article.
-
Heteroatom-Heteroatom Bond Formation in Natural Product Biosynthesis.Chem Rev. 2017 Apr 26;117(8):5784-5863. doi: 10.1021/acs.chemrev.6b00621. Epub 2017 Apr 4. Chem Rev. 2017. PMID: 28375000 Free PMC article. Review.
-
Glutamic acid is a carrier for hydrazine during the biosyntheses of fosfazinomycin and kinamycin.Nat Commun. 2018 Sep 11;9(1):3687. doi: 10.1038/s41467-018-06083-7. Nat Commun. 2018. PMID: 30206228 Free PMC article.
References
-
- Kuroda Y., Okuhara M., Goto T., Okamoto M., Terano H., Kohsaka M., Aoki H., Imanaka H. J. Antibiot. 1980;33:29–35. - PubMed
-
- Ogita T., Gunji S., Fukuzawa Y., Terahara A., Kinoshita T., Nagaki H., Beppu T. Tetrahedron Lett. 1983;24:2283–2286.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

