From dinosaurs to birds: a tail of evolution
- PMID: 25621146
- PMCID: PMC4304130
- DOI: 10.1186/2041-9139-5-25
From dinosaurs to birds: a tail of evolution
Abstract
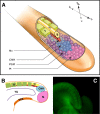
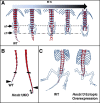
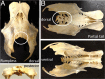
A particularly critical event in avian evolution was the transition from long- to short-tailed birds. Primitive bird tails underwent significant alteration, most notably reduction of the number of caudal vertebrae and fusion of the distal caudal vertebrae into an ossified pygostyle. These changes, among others, occurred over a very short evolutionary interval, which brings into focus the underlying mechanisms behind those changes. Despite the wealth of studies delving into avian evolution, virtually nothing is understood about the genetic and developmental events responsible for the emergence of short, fused tails. In this review, we summarize the current understanding of the signaling pathways and morphological events that contribute to tail extension and termination and examine how mutations affecting the genes that control these pathways might influence the evolution of the avian tail. To generate a list of candidate genes that may have been modulated in the transition to short-tailed birds, we analyzed a comprehensive set of mouse mutants. Interestingly, a prevalent pleiotropic effect of mutations that cause fused caudal vertebral bodies (as in the pygostyles of birds) is tail truncation. We identified 23 mutations in this class, and these were primarily restricted to genes involved in axial extension. At least half of the mutations that cause short, fused tails lie in the Notch/Wnt pathway of somite boundary formation or differentiation, leading to changes in somite number or size. Several of the mutations also cause additional bone fusions in the trunk skeleton, reminiscent of those observed in primitive and modern birds. All of our findings were correlated to the fossil record. An open question is whether the relatively sudden appearance of short-tailed birds in the fossil record could be accounted for, at least in part, by the pleiotropic effects generated by a relatively small number of mutational events.
Keywords: Archaeopteryx; Avian; Bird evolution; Confuciusornis; Dinosaur; Jeholornis; Sapeornis; Somitogenesis; Tail.
Figures





Similar articles
-
Avian tail ontogeny, pygostyle formation, and interpretation of juvenile Mesozoic specimens.Sci Rep. 2018 Jun 13;8(1):9014. doi: 10.1038/s41598-018-27336-x. Sci Rep. 2018. PMID: 29899503 Free PMC article.
-
FROM FROND TO FAN: ARCHAEOPTERYX AND THE EVOLUTION OF SHORT-TAILED BIRDS.Evolution. 1996 Oct;50(5):2037-2048. doi: 10.1111/j.1558-5646.1996.tb03590.x. Evolution. 1996. PMID: 28565606
-
Jeholornis compared to Archaeopteryx, with a new understanding of the earliest avian evolution.Naturwissenschaften. 2003 May;90(5):220-5. doi: 10.1007/s00114-003-0416-5. Epub 2003 Apr 15. Naturwissenschaften. 2003. PMID: 12743704
-
Air-filled postcranial bones in theropod dinosaurs: physiological implications and the 'reptile'-bird transition.Biol Rev Camb Philos Soc. 2012 Feb;87(1):168-93. doi: 10.1111/j.1469-185X.2011.00190.x. Epub 2011 Jul 7. Biol Rev Camb Philos Soc. 2012. PMID: 21733078 Review.
-
Bird evolution in the Eocene: climate change in Europe and a Danish fossil fauna.Biol Rev Camb Philos Soc. 2006 Nov;81(4):483-99. doi: 10.1017/S146479310600707X. Epub 2006 Aug 8. Biol Rev Camb Philos Soc. 2006. PMID: 16893476 Review.
Cited by
-
Development of the Synarcual in the Elephant Sharks (Holocephali; Chondrichthyes): Implications for Vertebral Formation and Fusion.PLoS One. 2015 Sep 4;10(9):e0135138. doi: 10.1371/journal.pone.0135138. eCollection 2015. PLoS One. 2015. PMID: 26339918 Free PMC article.
-
Experimental determination of three-dimensional cervical joint mobility in the avian neck.Front Zool. 2017 Jul 24;14:37. doi: 10.1186/s12983-017-0223-z. eCollection 2017. Front Zool. 2017. PMID: 28747987 Free PMC article.
-
Postnatal ossification sequences in Acrocephalus scirpaceus and Chroicocephalus ridibundus (Aves: Neognathae): The precocial-altricial spectrum and evolution of compound bones in birds.J Anat. 2021 Feb;238(2):349-364. doi: 10.1111/joa.13303. Epub 2020 Sep 1. J Anat. 2021. PMID: 32875600 Free PMC article.
-
Time course and side-by-side analysis of mesodermal, pre-myogenic, myogenic and differentiated cell markers in the chicken model for skeletal muscle formation.J Anat. 2015 Sep;227(3):361-82. doi: 10.1111/joa.12353. J Anat. 2015. PMID: 26278933 Free PMC article.
-
Avian tail ontogeny, pygostyle formation, and interpretation of juvenile Mesozoic specimens.Sci Rep. 2018 Jun 13;8(1):9014. doi: 10.1038/s41598-018-27336-x. Sci Rep. 2018. PMID: 29899503 Free PMC article.
References
-
- Chiappe LM, Witmer LM. Mesozoic Birds: Above the Heads of Dinosaurs. Berkeley, Los Angeles, and London: University of California Press; 2002.
-
- Chiappe LM. Glorified Dinosaurs: The Origin and Early Evolution of Birds. Sydney, Australia or Hoboken. NJ USA: University of New South Wales or John Wiley & Sons, Inc.; 2007.
-
- Gatesy SM. Caudofemoral musculature and the evolution of theropod locomotion. Paleobiology. 1990;16:170–186.
-
- Chiappe LM. In: Encyclopedia of Dinosaurs. Currie PJ, Padian K, editor. San Diego: Academic Press; 1997. Aves; pp. 32–38.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

