Bioenergetic shifts during transitions between stem cell states (2013 Grover Conference series)
- PMID: 25621152
- PMCID: PMC4278598
- DOI: 10.1086/677353
Bioenergetic shifts during transitions between stem cell states (2013 Grover Conference series)
Abstract
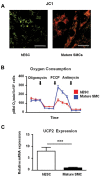
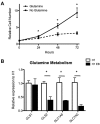
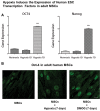
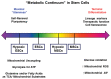
Two defining characteristics of stem cells are their multilineage differentiation potential (multipotency or pluripotency) and their capacity for self-renewal. Growth factors are well-established regulators of stem cell differentiation and self renewal, but less is known about the influence of the metabolic state on stem cell function. Recent studies investigating cellular metabolism during the differentiation of adult stem cells, human embryonic stem cells (ESCs), and induced pluripotent stem cells have demonstrated that activation of specific metabolic pathways depends on the type of stem cells as well as the lineage cells are differentiating into and that these metabolic pathways can influence the differentiation process. However, some common patterns have emerged, suggesting that undifferentiated stem cells primarily rely on glycolysis to meet energy demands. Our own data indicate that undifferentiated ESCs not only exhibit a low mitochondrial membrane potential but also express high levels of the mitochondrial uncoupling protein 2 and of glutamine metabolism regulators when compared with differentiated cells. More importantly, interventions that target stem cell metabolism are able to either prevent or enhance differentiation. These findings suggest that the metabolic state of stem cells is not just a marker of their differentiation status but also plays an active role in regulating stem cell function. Regulatory metabolic pathways in stem cells may thus serve as important checkpoints that can be modulated to direct the regenerative capacity of stem cells.
Keywords: UCP2; Warburg; differentiation; embryonic stem cells; glutamine; induced pluripotent stem cells; mesenchymal stem cells; metabolism; mitochondria; stem cells; uncoupling protein 2.
Figures
Similar articles
-
Mitochondrial bioenergetic function and metabolic plasticity in stem cell differentiation and cellular reprogramming.Biochim Biophys Acta. 2012 May;1820(5):571-6. doi: 10.1016/j.bbagen.2011.09.013. Epub 2011 Sep 29. Biochim Biophys Acta. 2012. PMID: 21983491 Review.
-
Mitochondria in mesenchymal stem cell biology and cell therapy: From cellular differentiation to mitochondrial transfer.Semin Cell Dev Biol. 2016 Apr;52:119-31. doi: 10.1016/j.semcdb.2016.02.011. Epub 2016 Feb 8. Semin Cell Dev Biol. 2016. PMID: 26868759 Review.
-
Revisiting Mitochondrial Function and Metabolism in Pluripotent Stem Cells: Where Do We Stand in Neurological Diseases?Mol Neurobiol. 2017 Apr;54(3):1858-1873. doi: 10.1007/s12035-016-9714-8. Epub 2016 Feb 18. Mol Neurobiol. 2017. PMID: 26892627 Review.
-
Mechanisms of the Metabolic Shift during Somatic Cell Reprogramming.Int J Mol Sci. 2019 May 7;20(9):2254. doi: 10.3390/ijms20092254. Int J Mol Sci. 2019. PMID: 31067778 Free PMC article. Review.
-
Bioenergetic Changes Underline Plasticity of Murine Embryonic Stem Cells.Stem Cells. 2019 Apr;37(4):463-475. doi: 10.1002/stem.2965. Epub 2019 Jan 24. Stem Cells. 2019. PMID: 30599083
Cited by
-
Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy.Stem Cells Int. 2016;2016:3516574. doi: 10.1155/2016/3516574. Epub 2016 Jan 10. Stem Cells Int. 2016. PMID: 26880954 Free PMC article.
-
Every Breath You Take: Non-invasive Real-Time Oxygen Biosensing in Two- and Three-Dimensional Microfluidic Cell Models.Front Physiol. 2018 Jul 3;9:815. doi: 10.3389/fphys.2018.00815. eCollection 2018. Front Physiol. 2018. PMID: 30018569 Free PMC article.
-
Oncostatic-Cytoprotective Effect of Melatonin and Other Bioactive Molecules: A Common Target in Mitochondrial Respiration.Int J Mol Sci. 2016 Mar 7;17(3):341. doi: 10.3390/ijms17030341. Int J Mol Sci. 2016. PMID: 26959015 Free PMC article. Review.
-
MetaboLINK is a novel algorithm for unveiling cell-specific metabolic pathways in longitudinal datasets.Front Neurosci. 2025 Jan 13;18:1520982. doi: 10.3389/fnins.2024.1520982. eCollection 2024. Front Neurosci. 2025. PMID: 39872998 Free PMC article.
-
Metabolic programming of nephron progenitor cell fate.Pediatr Nephrol. 2021 Aug;36(8):2155-2164. doi: 10.1007/s00467-020-04752-8. Epub 2020 Oct 21. Pediatr Nephrol. 2021. PMID: 33089379 Free PMC article. Review.
References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. - PubMed
-
- Xu X, Duan S, Yi F, Ocampo A, Liu GH, Izpisua Belmonte JC. Mitochondrial regulation in pluripotent stem cells. Cell Metab 2013:18:325–332. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

