Current and future medical therapeutic strategies for the functional repair of spinal cord injury
- PMID: 25621210
- PMCID: PMC4303789
- DOI: 10.5312/wjo.v6.i1.42
Current and future medical therapeutic strategies for the functional repair of spinal cord injury
Abstract
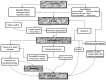
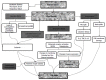
Spinal cord injury (SCI) leads to social and psychological problems in patients and requires costly treatment and care. In recent years, various pharmacological agents have been tested for acute SCI. Large scale, prospective, randomized, controlled clinical trials have failed to demonstrate marked neurological benefit in contrast to their success in the laboratory. Today, the most important problem is ineffectiveness of nonsurgical treatment choices in human SCI that showed neuroprotective effects in animal studies. Recently, attempted cellular therapy and transplantations are promising. A better understanding of the pathophysiology of SCI started in the early 1980s. Research had been looking at neuroprotection in the 1980s and the first half of 1990s and regeneration studies started in the second half of the 1990s. A number of studies on surgical timing suggest that early surgical intervention is safe and feasible, can improve clinical and neurological outcomes and reduce health care costs, and minimize the secondary damage caused by compression of the spinal cord after trauma. This article reviews current evidence for early surgical decompression and nonsurgical treatment options, including pharmacological and cellular therapy, as the treatment choices for SCI.
Keywords: Cellular treatment; Management; Pharmacological treatment; Spinal cord injury; Trauma; Treatment.
Figures
Similar articles
-
Current updates on various treatment approaches in the early management of acute spinal cord injury.Rev Neurosci. 2021 Feb 10;32(5):513-530. doi: 10.1515/revneuro-2020-0148. Print 2021 Jul 27. Rev Neurosci. 2021. PMID: 33565738
-
Neuroprotection and acute spinal cord injury: a reappraisal.NeuroRx. 2004 Jan;1(1):80-100. doi: 10.1602/neurorx.1.1.80. NeuroRx. 2004. PMID: 15717009 Free PMC article. Review.
-
Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury.Neurosurg Focus. 2008;25(5):E14. doi: 10.3171/FOC.2008.25.11.E14. Neurosurg Focus. 2008. PMID: 18980474 Review.
-
Cerebrolysin, a mixture of neurotrophic factors induces marked neuroprotection in spinal cord injury following intoxication of engineered nanoparticles from metals.CNS Neurol Disord Drug Targets. 2012 Feb;11(1):40-9. doi: 10.2174/187152712799960781. CNS Neurol Disord Drug Targets. 2012. PMID: 22229324 Review.
-
Efficacy of riluzole in the treatment of spinal cord injury: a systematic review of the literature.Neurosurg Focus. 2019 Mar 1;46(3):E6. doi: 10.3171/2019.1.FOCUS18596. Neurosurg Focus. 2019. PMID: 30835675
Cited by
-
Stem Cell Therapy in Spinal Cord Injury-Induced Neurogenic Lower Urinary Tract Dysfunction.Stem Cell Rev Rep. 2023 Aug;19(6):1691-1708. doi: 10.1007/s12015-023-10547-9. Epub 2023 Apr 28. Stem Cell Rev Rep. 2023. PMID: 37115409 Review.
-
The use of autologous neurogenically-induced bone marrow-derived mesenchymal stem cells for the treatment of paraplegic dogs without nociception due to spinal trauma.J Vet Med Sci. 2016 Oct 1;78(9):1465-1473. doi: 10.1292/jvms.15-0571. Epub 2016 Jun 10. J Vet Med Sci. 2016. PMID: 27301583 Free PMC article.
-
Clinical Neurorestorative Therapeutic Guidelines for Spinal Cord Injury (IANR/CANR version 2019).J Orthop Translat. 2019 Nov 11;20:14-24. doi: 10.1016/j.jot.2019.10.006. eCollection 2020 Jan. J Orthop Translat. 2019. PMID: 31908929 Free PMC article. Review.
-
Human umbilical cord mesenchymal stem cells-derived extracellular vesicles facilitate the repair of spinal cord injury via the miR-29b-3p/PTEN/Akt/mTOR axis.Cell Death Discov. 2021 Aug 11;7(1):212. doi: 10.1038/s41420-021-00572-3. Cell Death Discov. 2021. PMID: 34381025 Free PMC article.
-
Clemastine Promotes Differentiation of Oligodendrocyte Progenitor Cells Through the Activation of ERK1/2 via Muscarinic Receptors After Spinal Cord Injury.Front Pharmacol. 2022 Jul 5;13:914153. doi: 10.3389/fphar.2022.914153. eCollection 2022. Front Pharmacol. 2022. PMID: 35865954 Free PMC article.
References
-
- Aki T, Toya S. Experimental study on changes of the spinal-evoked potential and circulatory dynamics following spinal cord compression and decompression. Spine (Phila Pa 1976) 1984;9:800–809. - PubMed
-
- Tator CH, Edmonds VE. Acute spinal cord injury: analysis of epidemiologic factors. Can J Surg. 1979;22:575–578. - PubMed
-
- Karacan I, Koyuncu H, Pekel O, Sümbüloglu G, Kirnap M, Dursun H, Kalkan A, Cengiz A, Yalinkiliç A, Unalan HI, et al. Traumatic spinal cord injuries in Turkey: a nation-wide epidemiological study. Spinal Cord. 2000;38:697–701. - PubMed
-
- Citterio A, Franceschini M, Spizzichino L, Reggio A, Rossi B, Stampacchia G. Nontraumatic spinal cord injury: an Italian survey. Arch Phys Med Rehabil. 2004;85:1483–1487. - PubMed
-
- Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. JAMA. 1911;57:878–880.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

