Polyethylenimine-mediated expression of transgenes in the acinar cells of rats salivary glands in vivo
- PMID: 25621283
- PMCID: PMC4288386
- DOI: 10.3389/fcell.2014.00074
Polyethylenimine-mediated expression of transgenes in the acinar cells of rats salivary glands in vivo
Abstract
Non viral-mediated transfection of plasmid DNA provides a fast and reliable way to express various transgenes in selected cell populations in live animals. Here, we show an improvement of a previously published method that is based on injecting plasmid DNA into the ductal system of the salivary glands in live rats. Specifically, using complexes between plasmid DNA and polyethyleneimine (PEI) we show that the expression of the transgenes is directed selectively to the salivary acinar cells. PEI does not affect the ability of cells to undergo regulated exocytosis, which was one of the main drawbacks of the previous methods. Moreover PEI does not affect the proper localization and targeting of transfected proteins, as shown for the apical plasma membrane water channel aquaporin 5 (AQP5). Overall, this approach, coupled with the use of intravital microscopy, permits to conduct localization and functional studies under physiological conditions, in a rapid, reliable, and affordable fashion.
Keywords: acinar cells; aquaporin 5; in vivo transfection; intravital microscopy; non-viral gene delivery; polyethyleneimine; salivary glands.
Figures
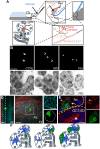
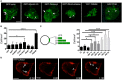
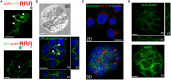
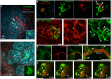
Similar articles
-
Plasmid DNA is internalized from the apical plasma membrane of the salivary gland epithelium in live animals.Histochem Cell Biol. 2012 Aug;138(2):201-13. doi: 10.1007/s00418-012-0959-7. Epub 2012 Apr 29. Histochem Cell Biol. 2012. PMID: 22544351 Free PMC article.
-
Immunolocalization of the water channel, aquaporin-5 (AQP5), in the rat digestive system.Arch Histol Cytol. 2003 Oct;66(4):307-15. doi: 10.1679/aohc.66.307. Arch Histol Cytol. 2003. PMID: 14692686
-
Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: immunolocalization and effect of secretory stimulation.Cell Tissue Res. 1999 Mar;295(3):513-21. doi: 10.1007/s004410051257. Cell Tissue Res. 1999. PMID: 10022971
-
Physiological role of aquaporin 5 in salivary glands.Pflugers Arch. 2016 Apr;468(4):519-39. doi: 10.1007/s00424-015-1749-6. Epub 2015 Nov 5. Pflugers Arch. 2016. PMID: 26537593 Review.
-
Aquaporin water channel in salivary glands.Jpn J Pharmacol. 2000 Jun;83(2):95-101. doi: 10.1254/jjp.83.95. Jpn J Pharmacol. 2000. PMID: 10928320 Review.
Cited by
-
Imaging membrane remodeling during regulated exocytosis in live mice.Exp Cell Res. 2015 Oct 1;337(2):219-25. doi: 10.1016/j.yexcr.2015.06.018. Epub 2015 Jul 6. Exp Cell Res. 2015. PMID: 26160452 Free PMC article. Review.
-
Endocytosis in gene therapy with non-viral vectors.Wien Med Wochenschr. 2016 May;166(7-8):227-35. doi: 10.1007/s10354-016-0450-5. Epub 2016 May 3. Wien Med Wochenschr. 2016. PMID: 27141862 Review. English.
-
Intravital microscopy in mammalian multicellular organisms.Curr Opin Cell Biol. 2019 Aug;59:97-103. doi: 10.1016/j.ceb.2019.03.015. Epub 2019 May 21. Curr Opin Cell Biol. 2019. PMID: 31125832 Free PMC article. Review.
References
-
- Boulaiz H., Marchal J. A., Prados J., Melguizo C., Aranega A. (2005). Non-viral and viral vectors for gene therapy. Cell. Mol. Biol. (Noisy-le-grand). 51, 3–22. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

