Cordycepin is a novel chemical suppressor of Epstein-Barr virus replication
- PMID: 25621301
- PMCID: PMC4303894
- DOI: 10.18632/oncoscience.110
Cordycepin is a novel chemical suppressor of Epstein-Barr virus replication
Abstract
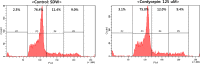
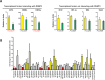
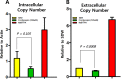
Cordyceps species are known to produce numerous active components and are used for diverse medicinal purposes because of their varied physiological activities, including their ability to protect the liver from damage as well as their anticancer, antidepressant, anti-inflammatory, hypoglycemic, antimicrobial effects. Cordycepin, an adenosine derivative, differs from adenosine in that its ribose lacks an oxygen atom at the 3' position. Several research groups have reported that cordycepin has antiviral activity against several viruses including influenza virus, plant viruses, human immunodeficiency virus(HIV), murine leukemia virus, and Epstein-Barr virus (EBV). In this study, we identify the epigenetic mechanisms by which cordycepin exerts its anti-gammaherpesvirus effects. We show that cordycepin possesses antitumor and antiviral activity against gastric carcinoma and EBV, respectively. A comparison of the CD50 values of cordycepin and its analogs showed that the lack of a 2'-hydroxyl group in cordycepin was critical for its relatively potent cytotoxicity. Cordycepin treatment decreased the rate of early apoptosis in SNU719 cells by up to 64%, but increased late apoptosis/necrosis by up to 31%. Interestingly, cordycepin increased BCL7A methylation in SNU719 cells by up to 58% and decreased demethylation by up to 37%. Consistent with these changes in methylation, cordycepin treatment significantly downregulated most EBV genes tested. Under the same conditions, cordycepin significantly decreased the frequency of Q and F promoter usage, and H3K4me3 histone enrichment was significantly reduced at several important EBV genomic loci. Extracellular and intracellular EBV genome copy numbers were reduced by up to 55% and 30%, respectively, in response to 125 μM cordycepin treatment. Finally, cordycepin significantly suppressed the transfer of EBV from LCL-EBV-GFP to AGS cells, indicating that EBV infection of gastric epithelial cells was inhibited. These results suggest that cordycepin has antiviral and antitumor activities against gammaherpesviruses and host cells latently infected with virus.
Keywords: Cordycepin; Epstein–Barr virus; antiviral agent; gastric carcinoma.
Figures








Similar articles
-
Adenosine Induces EBV Lytic Reactivation through ADORA1 in EBV-Associated Gastric Carcinoma.Int J Mol Sci. 2019 Mar 14;20(6):1286. doi: 10.3390/ijms20061286. Int J Mol Sci. 2019. PMID: 30875759 Free PMC article.
-
Genipin as a novel chemical activator of EBV lytic cycle.J Microbiol. 2015 Feb;53(2):155-65. doi: 10.1007/s12275-015-4672-9. Epub 2015 Jan 28. J Microbiol. 2015. PMID: 25626372
-
Cordycepin enhances Epstein-Barr virus lytic infection and Epstein-Barr virus-positive tumor treatment efficacy by doxorubicin.Cancer Lett. 2016 Jul 1;376(2):240-8. doi: 10.1016/j.canlet.2016.04.001. Epub 2016 Apr 7. Cancer Lett. 2016. PMID: 27063964
-
Gastritis-Infection-Cancer Sequence of Epstein-Barr Virus-Associated Gastric Cancer.Adv Exp Med Biol. 2018;1045:437-457. doi: 10.1007/978-981-10-7230-7_20. Adv Exp Med Biol. 2018. PMID: 29896679 Review.
-
DNA hypermethylation induced by Epstein-Barr virus in the development of Epstein-Barr virus-associated gastric carcinoma.Arch Pharm Res. 2017 Aug;40(8):894-905. doi: 10.1007/s12272-017-0939-5. Epub 2017 Aug 4. Arch Pharm Res. 2017. PMID: 28779374 Review.
Cited by
-
Review of Naturopathy of Medical Mushroom, Ophiocordyceps Sinensis, in Sexual Dysfunction.Pharmacogn Rev. 2016 Jan-Jun;10(19):1-5. doi: 10.4103/0973-7847.176566. Pharmacogn Rev. 2016. PMID: 27041868 Free PMC article. Review.
-
Therapies based on targeting Epstein-Barr virus lytic replication for EBV-associated malignancies.Cancer Sci. 2018 Jul;109(7):2101-2108. doi: 10.1111/cas.13634. Epub 2018 Jun 13. Cancer Sci. 2018. PMID: 29751367 Free PMC article. Review.
-
Cordycepin suppresses the migration and invasion of human liver cancer cells by downregulating the expression of CXCR4.Int J Mol Med. 2020 Jan;45(1):141-150. doi: 10.3892/ijmm.2019.4391. Epub 2019 Oct 31. Int J Mol Med. 2020. PMID: 31746344 Free PMC article.
-
Ergosterol peroxide activates Foxo3-mediated cell death signaling by inhibiting AKT and c-Myc in human hepatocellular carcinoma cells.Oncotarget. 2016 Jun 7;7(23):33948-59. doi: 10.18632/oncotarget.8608. Oncotarget. 2016. PMID: 27058618 Free PMC article.
-
Cordycepin Inhibits Enterovirus A71 Replication and Protects Host Cell from Virus-Induced Cytotoxicity through Adenosine Action Pathway.Viruses. 2024 Feb 24;16(3):352. doi: 10.3390/v16030352. Viruses. 2024. PMID: 38543718 Free PMC article.
References
-
- Das SK, Masuda M, Sakurai A, Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia. 2010;81(8):961–968. - PubMed
-
- Gu YX, Song YW, Fan LQ, Yuan QS. [Antioxidant activity of natural and cultured Cordyceps sp] Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 2007;32(11):1028–1031. - PubMed
-
- Pharmacognosy Cotpi. Pharmacognosy-Pharmacognosy. Seoul Korea: Dong Myoung; 2013. pp. 701–705.
-
- Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. The Journal of pharmacy and pharmacology. 2005;57(12):1509–1519. - PubMed
-
- Nakamura K, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Activation of in vivo Kupffer cell function by oral administration of Cordyceps sinensis in rats. Japanese journal of pharmacology. 1999;79(4):505–508. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

