Bis-aryl urea derivatives as potent and selective LIM kinase (Limk) inhibitors
- PMID: 25621531
- PMCID: PMC4349585
- DOI: 10.1021/jm501680m
Bis-aryl urea derivatives as potent and selective LIM kinase (Limk) inhibitors
Abstract
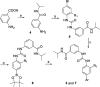
The discovery/optimization of bis-aryl ureas as Limk inhibitors to obtain high potency and selectivity and appropriate pharmacokinetic properties through systematic SAR studies is reported. Docking studies supported the observed SAR. Optimized Limk inhibitors had high biochemical potency (IC50 < 25 nM), excellent selectivity against ROCK and JNK kinases (>400-fold), potent inhibition of cofilin phosphorylation in A7r5, PC-3, and CEM-SS T cells (IC50 < 1 μM), and good in vitro and in vivo pharmacokinetic properties. In the profiling against a panel of 61 kinases, compound 18b at 1 μM inhibited only Limk1 and STK16 with ≥80% inhibition. Compounds 18b and 18f were highly efficient in inhibiting cell-invasion/migration in PC-3 cells. In addition, compound 18w was demonstrated to be effective on reducing intraocular pressure (IOP) on rat eyes. Taken together, these data demonstrated that we had developed a novel class of bis-aryl urea derived potent and selective Limk inhibitors.
Figures







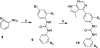


References
-
- Bernard O, Ganiatsas S, Kannourakis G, Dringen R. Kiz-1, a protein with LIM zinc finger and kinase domains, is expressed mainly in neurons. Cell Growth Differ. 1994;5:1159–1171. - PubMed
-
- Stanyon CA, Bernard O. LIM-kinase1. Int J Biochem Cell Biol. 1999;31:389–394. - PubMed
-
- Mizuno K, Okana I, Ohashi K, Nunoue K, Kuma K, Miyata T, Nakamura T. Identification of a human cDNA encoding a novel protein kinase with two repeats of the LIM/double Zinc finger motif. Oncogene. 1994;9:1605–1612. - PubMed
-
- Osada H, Hasada K, Inazawa J, Uchida K, Ueda R, Takahashi T, Takahashi T. Subcellular localization and protein interaction of the human LIMK2 gene expressing alternative transcripts with tissue-specific regulation. Biochem Biophys Res Commun. 1996;229:582–589. - PubMed
-
- Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol. 2007;39:1071–1076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Research Materials
Miscellaneous

