Protein glycosylation in cancer
- PMID: 25621663
- PMCID: PMC4396820
- DOI: 10.1146/annurev-pathol-012414-040438
Protein glycosylation in cancer
Abstract
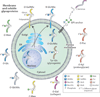
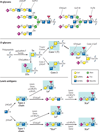
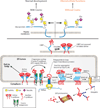
Neoplastic transformation results in a wide variety of cellular alterations that impact the growth, survival, and general behavior of affected tissue. Although genetic alterations underpin the development of neoplastic disease, epigenetic changes can exert an equally significant effect on neoplastic transformation. Among neoplasia-associated epigenetic alterations, changes in cellular glycosylation have recently received attention as a key component of neoplastic progression. Alterations in glycosylation appear to not only directly impact cell growth and survival but also facilitate tumor-induced immunomodulation and eventual metastasis. Many of these changes may support neoplastic progression, and unique alterations in tumor-associated glycosylation may also serve as a distinct feature of cancer cells and therefore provide novel diagnostic and even therapeutic targets.
Keywords: biomarkers; cancer; glycans; glycoprotein; glycosylation; immunohistochemistry; oligosaccharides; transformation.
Figures


References
-
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. - PubMed
-
- Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat. Rev. Cancer. 2005;5:526–542. - PubMed
-
- Jones PA, Laird PW. Cancer epigenetics comes of age. Nat. Genet. 1999;21:163–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

