Designing chimeric antigen receptors to effectively and safely target tumors
- PMID: 25621840
- PMCID: PMC4397136
- DOI: 10.1016/j.coi.2015.01.002
Designing chimeric antigen receptors to effectively and safely target tumors
Abstract
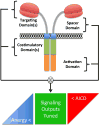
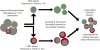
The adoptive transfer of T cells engineered to express artificial chimeric antigen receptors CARs) that target a tumor cell surface molecule has emerged as an exciting new approach for cancer immunotherapy. Clinical trials in patients with advanced B cell malignancies treated with CD19-specific CAR-modified T cells (CAR-T) have shown impressive antitumor efficacy, leading to optimism that this approach will be useful for treating common solid tumors. Because CAR-T cells recognize tumor cells independent of their expression of human leukocyte antigen (HLA) molecules, tumors that escape conventional T cells by downregulating HLA and/or mutating components of the antigen processing machinery can be eliminated. The ability to introduce or delete additional genes in T cells has the potential to provide therapeutic cell products with novel attributes that overcome impediments to immune mediated tumor elimination in immunosuppressive tumor microenvironments. This review will discuss recent concepts in the development of effective and safe synthetic CARs for adoptive T cell therapy (ACT).
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
References
-
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. 2014;6:224ra225. This paper describes the results of treating advanced acute lymphoblastic leukemia with autologous T cells modified with a CD19-specific CAR containing the CD28 and CD3ζ signaling domains. An algorithm for identifying and managing patients with cytokine release syndrome is provided. - PMC - PubMed
-
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated With Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 - PMC - PubMed
-
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. The study reports results in pediatric acute lymphoblastic leukemia demonstrating that treatment with CD19 specific chimeric antigen receptor-modified T cells against CD19 was was associated with a high remission rate, even among patients for whom stem-cell transplantation had failed. - PMC - PubMed
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2014 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

