Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection
- PMID: 25621893
- PMCID: PMC4306481
- DOI: 10.1371/journal.ppat.1004589
Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection
Abstract
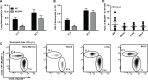
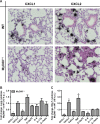
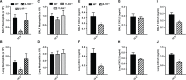
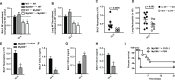
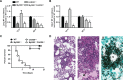
Aspergillus fumigatus forms ubiquitous airborne conidia that humans inhale on a daily basis. Although respiratory fungal infection activates the adaptor proteins CARD9 and MyD88 via C-type lectin, Toll-like, and interleukin-1 family receptor signals, defining the temporal and spatial pattern of MyD88- and CARD9-coupled signals in immune activation and fungal clearance has been difficult to achieve. Herein, we demonstrate that MyD88 and CARD9 act in two discrete phases and in two cellular compartments to direct chemokine- and neutrophil-dependent host defense. The first phase depends on MyD88 signaling because genetic deletion of MyD88 leads to delayed induction of the neutrophil chemokines CXCL1 and CXCL5, delayed neutrophil lung trafficking, and fatal pulmonary damage at the onset of respiratory fungal infection. MyD88 expression in lung epithelial cells restores rapid chemokine induction and neutrophil recruitment via interleukin-1 receptor signaling. Exogenous CXCL1 administration reverses murine mortality in MyD88-deficient mice. The second phase depends predominately on CARD9 signaling because genetic deletion of CARD9 in radiosensitive hematopoietic cells interrupts CXCL1 and CXCL2 production and lung neutrophil recruitment beyond the initial MyD88-dependent phase. Using a CXCL2 reporter mouse, we show that lung-infiltrating neutrophils represent the major cellular source of CXCL2 during CARD9-dependent recruitment. Although neutrophil-intrinsic MyD88 and CARD9 function are dispensable for neutrophil conidial uptake and killing in the lung, global deletion of both adaptor proteins triggers rapidly progressive invasive disease when mice are challenged with an inoculum that is sub-lethal for single adapter protein knockout mice. Our findings demonstrate that distinct signal transduction pathways in the respiratory epithelium and hematopoietic compartment partially overlap to ensure optimal chemokine induction, neutrophil recruitment, and fungal clearance within the respiratory tract.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
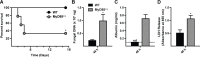


Similar articles
-
CARD9+ microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment.Nat Immunol. 2019 May;20(5):559-570. doi: 10.1038/s41590-019-0377-2. Epub 2019 Apr 17. Nat Immunol. 2019. PMID: 30996332 Free PMC article.
-
Myeloid differentiation factor 88-dependent signaling is critical for acute organic dust-induced airway inflammation in mice.Am J Respir Cell Mol Biol. 2013 Jun;48(6):781-9. doi: 10.1165/rcmb.2012-0479OC. Am J Respir Cell Mol Biol. 2013. PMID: 23492189 Free PMC article.
-
During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk.Cell Host Microbe. 2020 Jul 8;28(1):104-116.e4. doi: 10.1016/j.chom.2020.05.002. Epub 2020 Jun 1. Cell Host Microbe. 2020. PMID: 32485165 Free PMC article.
-
Inherited CARD9 Deficiency: Invasive Disease Caused by Ascomycete Fungi in Previously Healthy Children and Adults.J Clin Immunol. 2018 Aug;38(6):656-693. doi: 10.1007/s10875-018-0539-2. Epub 2018 Aug 22. J Clin Immunol. 2018. PMID: 30136218 Free PMC article. Review.
-
Mechanistic Insights into the Role of C-Type Lectin Receptor/CARD9 Signaling in Human Antifungal Immunity.Front Cell Infect Microbiol. 2016 Apr 5;6:39. doi: 10.3389/fcimb.2016.00039. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27092298 Free PMC article. Review.
Cited by
-
Paving the way for predictive diagnostics and personalized treatment of invasive aspergillosis.Front Microbiol. 2015 May 5;6:411. doi: 10.3389/fmicb.2015.00411. eCollection 2015. Front Microbiol. 2015. PMID: 25999936 Free PMC article. Review.
-
Could the Lung Be a Gateway for Amphotericin B to Attack the Army of Fungi?Pharmaceutics. 2022 Dec 3;14(12):2707. doi: 10.3390/pharmaceutics14122707. Pharmaceutics. 2022. PMID: 36559201 Free PMC article. Review.
-
Molecular and physiological roles of the adaptor protein CARD9 in immunity.Cell Death Dis. 2018 Jan 19;9(2):52. doi: 10.1038/s41419-017-0084-6. Cell Death Dis. 2018. PMID: 29352133 Free PMC article. Review.
-
Cellular and functional heterogeneity of the airway epithelium.Mucosal Immunol. 2021 Sep;14(5):978-990. doi: 10.1038/s41385-020-00370-7. Epub 2021 Feb 19. Mucosal Immunol. 2021. PMID: 33608655 Free PMC article. Review.
-
Candidiasis of the Central Nervous System in Neonates and Children with Primary Immunodeficiencies.Curr Fungal Infect Rep. 2018 Jun;12(2):92-97. doi: 10.1007/s12281-018-0316-y. Epub 2018 May 8. Curr Fungal Infect Rep. 2018. PMID: 30393511 Free PMC article.
References
-
- Latge JP (2001) The pathobiology of Aspergillus fumigatus. Trends Microbiol 9: 382–389. - PubMed
-
- Schaffner A, Douglas H, Braude A (1982) Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest 69: 617–631. 10.1172/JCI110489 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

