Natural killer cells limit cardiac inflammation and fibrosis by halting eosinophil infiltration
- PMID: 25622543
- PMCID: PMC4348473
- DOI: 10.1016/j.ajpath.2014.11.023
Natural killer cells limit cardiac inflammation and fibrosis by halting eosinophil infiltration
Abstract
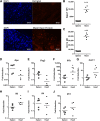
Myocarditis is a leading cause of sudden cardiac failure in young adults. Natural killer (NK) cells, a subset of the innate lymphoid cell compartment, are protective in viral myocarditis. Herein, we demonstrated that these protective qualities extend to suppressing autoimmune inflammation. Experimental autoimmune myocarditis (EAM) was initiated in BALB/c mice by immunization with myocarditogenic peptide. During EAM, activated cardiac NK cells secreted interferon γ, perforin, and granzyme B, and expressed CD69, tumor necrosis factor-related apoptosis-inducing ligand treatment, and CD27 on their cell surfaces. The depletion of NK cells during EAM with anti-asialo GM1 antibody significantly increased myocarditis severity, and was accompanied by elevated fibrosis and a 10-fold increase in the percentage of cardiac-infiltrating eosinophils. The resultant influx of eosinophils to the heart was directly responsible for the increased disease severity in the absence of NK cells, because treatment with polyclonal antibody asialogangloside GM-1 did not augment myocarditis severity in eosinophil-deficient ΔdoubleGATA1 mice. We demonstrate that NK cells limit eosinophilic infiltration both indirectly, through altering eosinophil-related chemokine production by cardiac fibroblasts, and directly, by inducing eosinophil apoptosis in vitro. Altogether, we define a new pathway of eosinophilic regulation through interactions with NK cells.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





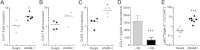

Similar articles
-
Macrophages and cardiac fibroblasts are the main producers of eotaxins and regulate eosinophil trafficking to the heart.Eur J Immunol. 2016 Dec;46(12):2749-2760. doi: 10.1002/eji.201646557. Epub 2016 Oct 25. Eur J Immunol. 2016. PMID: 27621211 Free PMC article.
-
CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis.Circ Res. 2009 Mar 13;104(5):628-38. doi: 10.1161/CIRCRESAHA.108.192179. Epub 2009 Jan 22. Circ Res. 2009. PMID: 19168435
-
Fatal eosinophilic myocarditis develops in the absence of IFN-γ and IL-17A.J Immunol. 2013 Oct 15;191(8):4038-47. doi: 10.4049/jimmunol.1301282. Epub 2013 Sep 18. J Immunol. 2013. PMID: 24048893 Free PMC article.
-
From infection to autoimmunity.J Autoimmun. 2001 May;16(3):175-86. doi: 10.1006/jaut.2000.0492. J Autoimmun. 2001. PMID: 11334481 Review.
-
Innate Immunity Effector Cells as Inflammatory Drivers of Cardiac Fibrosis.Int J Mol Sci. 2020 Sep 28;21(19):7165. doi: 10.3390/ijms21197165. Int J Mol Sci. 2020. PMID: 32998408 Free PMC article. Review.
Cited by
-
Immune Cells and Immunotherapy for Cardiac Injury and Repair.Circ Res. 2021 May 28;128(11):1766-1779. doi: 10.1161/CIRCRESAHA.121.318005. Epub 2021 May 27. Circ Res. 2021. PMID: 34043424 Free PMC article. Review.
-
Autoimmunity in Acute Myocarditis: How Immunopathogenesis Steers New Directions for Diagnosis and Treatment.Curr Cardiol Rep. 2020 Mar 20;22(5):28. doi: 10.1007/s11886-020-01278-1. Curr Cardiol Rep. 2020. PMID: 32198622 Free PMC article. Review.
-
Early IFN-α signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19.Immunity. 2021 Nov 9;54(11):2650-2669.e14. doi: 10.1016/j.immuni.2021.09.002. Epub 2021 Sep 4. Immunity. 2021. PMID: 34592166 Free PMC article.
-
Circulating T-Cell Subsets, Monocytes, and Natural Killer Cells in Peripartum Cardiomyopathy: Results From the Multicenter IPAC Study.J Card Fail. 2018 Jan;24(1):33-42. doi: 10.1016/j.cardfail.2017.10.012. Epub 2017 Oct 24. J Card Fail. 2018. PMID: 29079307 Free PMC article.
-
The immunology of diabetic cardiomyopathy.Front Endocrinol (Lausanne). 2025 Apr 7;16:1542208. doi: 10.3389/fendo.2025.1542208. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40260277 Free PMC article. Review.
References
-
- Andreoletti L., Leveque N., Boulagnon C., Brasselet C., Fornes P. Viral causes of human myocarditis. Arch Cardiovasc Dis. 2009;102:559–568. - PubMed
-
- Cihakova D., Rose N.R. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008;99:95–114. - PubMed
-
- Rose N.R., Cihakova D. Cardiomyopathies. Autoimmunity. 2004;37:347–350. - PubMed
-
- Seshadri S., Narula J., Chopra P. Asymptomatic eosinophilic myocarditis: 2 + 2 = 4 or 5! Int J Cardiol. 1991;31:348–349. - PubMed
-
- Huston B., Froloff V., Mills K., McGee M. Death due to eosinophilic necrotizing myocarditis despite steroid treatment. Am J Forensic Med Pathol. 2013;34:95–97. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

