The PDZ3 domain of the cellular scaffolding protein MAGI-1 interacts with the Coxsackievirus and adenovirus receptor (CAR)
- PMID: 25622559
- PMCID: PMC4359947
- DOI: 10.1016/j.biocel.2015.01.012
The PDZ3 domain of the cellular scaffolding protein MAGI-1 interacts with the Coxsackievirus and adenovirus receptor (CAR)
Abstract
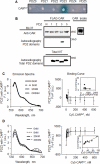
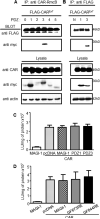
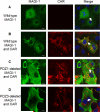
The Coxsackievirus and adenovirus receptor (CAR) is an essential cellular protein that is involved in cell-cell adhesion, protein trafficking, and viral infection. The major isoform of CAR is selectively sorted to the basolateral membrane of polarized epithelial cells where it co-localizes with the cellular scaffolding protein membrane-associated guanylate kinase with inverted domain structure-1 (MAGI-1). Previously, we demonstrated CAR interacts with MAGI-1 through a PDZ-domain dependent interaction. Here, we show that the PDZ3 domain of MAGI-1 is exclusively responsible for the high affinity interaction between the seven exon isoform of CAR and MAGI-1 using yeast-two-hybrid analysis and confirming this interaction biochemically and in cellular lysates by in vitro pull down assay and co-immunoprecipitation. The high affinity interaction between the PDZ3 domain and CAR C-terminus was measured by fluorescence resonance energy transfer. Further, we investigated the biological relevance of this high affinity interaction between CAR and the PDZ3 domain of MAGI-1 and found that it does not alter CAR-mediated adenovirus infection. By contrast, interruption of this high affinity interaction altered the localization of MAGI-1 indicating that CAR is able to traffic MAGI-1 to cell junctions. These data deepen the molecular understanding of the interaction between CAR and MAGI-1 and indicate that although CAR plays a role in trafficking PDZ-based scaffolding proteins to cellular junctions, association with a high affinity intracellular binding partner does not significantly alter adenovirus binding and entry via CAR.
Keywords: Adenovirus; Cell junction; Epithelia; PDZ domain.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
The PDZ1 and PDZ3 domains of MAGI-1 regulate the eight-exon isoform of the coxsackievirus and adenovirus receptor.J Virol. 2012 Sep;86(17):9244-54. doi: 10.1128/JVI.01138-12. Epub 2012 Jun 20. J Virol. 2012. PMID: 22718816 Free PMC article.
-
MAGI-1 PDZ2 Domain Blockade Averts Adenovirus Infection via Enhanced Proteolysis of the Apical Coxsackievirus and Adenovirus Receptor.J Virol. 2021 Jun 10;95(13):e0004621. doi: 10.1128/JVI.00046-21. Epub 2021 Jun 10. J Virol. 2021. PMID: 33762416 Free PMC article.
-
A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth.J Cell Sci. 2004 Sep 1;117(Pt 19):4401-9. doi: 10.1242/jcs.01300. Epub 2004 Aug 10. J Cell Sci. 2004. PMID: 15304526
-
The magic of MAGI-1: A scaffolding protein with multi signalosomes and functional plasticity.Biol Cell. 2022 Jul;114(7):185-198. doi: 10.1111/boc.202200014. Epub 2022 Apr 20. Biol Cell. 2022. PMID: 35389514 Review.
-
The coxsackievirus and adenovirus receptor: virological and biological beauty.FEBS Lett. 2020 Jun;594(12):1828-1837. doi: 10.1002/1873-3468.13794. Epub 2020 May 9. FEBS Lett. 2020. PMID: 32298477 Review.
Cited by
-
Adenovirus transduction: More complicated than receptor expression.Virology. 2017 Feb;502:144-151. doi: 10.1016/j.virol.2016.12.020. Epub 2016 Dec 31. Virology. 2017. PMID: 28049062 Free PMC article.
-
Isoform specific editing of the coxsackievirus and adenovirus receptor.Virology. 2019 Oct;536:20-26. doi: 10.1016/j.virol.2019.07.018. Epub 2019 Jul 27. Virology. 2019. PMID: 31394408 Free PMC article.
-
Poliovirus Receptor: More than a simple viral receptor.Virus Res. 2017 Oct 15;242:1-6. doi: 10.1016/j.virusres.2017.09.001. Epub 2017 Sep 8. Virus Res. 2017. PMID: 28870470 Free PMC article. Review.
-
L290P/V mutations increase ERK3's cytoplasmic localization and migration/invasion-promoting capability in cancer cells.Sci Rep. 2017 Nov 3;7(1):14979. doi: 10.1038/s41598-017-15135-9. Sci Rep. 2017. PMID: 29101390 Free PMC article.
-
CXADR: From an Essential Structural Component to a Vital Signaling Mediator in Spermatogenesis.Int J Mol Sci. 2023 Jan 9;24(2):1288. doi: 10.3390/ijms24021288. Int J Mol Sci. 2023. PMID: 36674801 Free PMC article. Review.
References
-
- Andersson B, Tomko Richard P., Edwards K, Mirza M, Darban H, Öncü D, Sonnhammer E, Sollerbrant K, Philipson L. Putative regulatory domains in the human and mouse CVADR genes. Gene Function & Disease. 2000;1(2):82–86.
-
- Asher DR, Cerny AM, Weiler SR, Horner JW, Keeler ML, Neptune MA, Jones SN, Bronson RT, Depinho RA, Finberg RW. Coxsackievirus and adenovirus receptor is essential for cardiomyocyte development. Genesis. 2005;42(2):77–85. - PubMed
-
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320–1323. - PubMed
-
- Bewley MC, Springer K, Zhang YB, Freimuth P, Flanagan JM. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science. 1999;286(5444):1579–1583. - PubMed
-
- Carson SD, Chapman NN, Tracy SM. Purification of the putative coxsackievirus B receptor from HeLa cells. Biochem Biophys Res Commun. 1997;233(2):325–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

