The state of point-of-care testing: a European perspective
- PMID: 25622619
- PMCID: PMC4389002
- DOI: 10.3109/03009734.2015.1006347
The state of point-of-care testing: a European perspective
Abstract
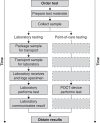
Point-of-care testing (POCT) refers to any diagnostic test administered outside the central laboratory at or near the location of the patient. By performing the sample collection and data analysis steps in the same location POCT cuts down on transport and processing delays, resulting in the rapid feedback of test results to medical decision-makers. Over the past decades the availability and use of POCT have steadily increased in Europe and throughout the international community. However, concerns about overall utility and the reliability of benefits to patient care have impeded the growth of POCT in some areas. While there is no agreed-upon standard for how success should be judged, the increases in speed and mobility provided by POCT can lead to substantial advantages over traditional laboratory testing. When properly utilized, POCT has been shown to yield measurable improvements in patient care, workflow efficiency, and even provide significant financial benefits. However, important organizational and quality assurance challenges must be addressed with the implementation of POCT in any health care environment. To ensure maximal benefits it may be necessary to evaluate critically and restructure existing clinical pathways to capitalize better on the rapid test turnaround times provided by POCT.
Keywords: laboratory analyses; near patient testing; outcome measurements; point-of-care.
Figures
References
-
- European Commission Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Brussels, Belgium: European Commission 1998. http://ec.europa.eu/enterprise/policies/european-standards/harmonised-st... Available from. Last accessed 7 December 1998.
-
- International Organization for Standardization Point of care (POC): Requirements for quality and competence (ISO 22870:2006). Geneva, Switzerland: International Organization for Standardization 2006. http://www.iso.org/iso/catalogue_detail.htm?csnumber=35173. Available from. Last accessed 01 February 2006.
-
- International Organization for Standardization Medical laboratories: Particular requirements for quality and competence (ISO 15189:2012). Geneva, Switzerland: International Organization for Standardization 2012. http://www.iso.org/iso/home/store/catalogue_ics/catalogue_detail_ics.htm.... Available from. Last accessed 01 November 2012.
-
- Scalise D. Poised for growth. point-of-care testing . Hosp Health Netw. 2006;80:77–83. - PubMed
-
- Kendall J, Reeves B, Clancy M. Point of care testing: randomised controlled trial of clinical outcome . BMJ. 1998;316:1052–7. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous